Objetivo: Evaluar la respuesta antiproteinúrica a un tratamiento multifactorial basado en dosis elevadas de antagonistas de los receptores de la angiotensina II (ARAII) (olmesartán) en pacientes con nefropatías proteinúricas no diabéticas, según tres de los polimorfismos del sistema renina-angiotensina (SRA): inserción/deleción del gen de la enzima convertidora de angiontensina (ECA), M235T del gen del angiotensinógeno y A1166C del receptor AT1 (rAT1) para la angiotensina II. Material y métodos: Se estudiaron 53 pacientes con nefropatía proteinúrica no diabética, con un tiempo medio de evolución de 84,4 ± 15 meses. Género varón en 41 (77,4 %); edad media 49,7 ± 3 años; índice de masa corporal 30 ± 6 kg/m2. Todos recibieron olmesartán (40 mg/12 h) asociado a un promedio de 2,4 ± 1,6 fármacos antihipertensivos durante una mediana de tiempo de 13 meses (rango intercuartil 7-25 meses). Resultados: La presión arterial (PA) sistólica descendió de 145 ± 14 hasta 128 ± 14 mmHg (p < 0,001) y la PA diastólica desde 85 ± 11 a 79 ± 7 mmHg (p < 0,01). La presión de pulso pasó de 53,5 ± 14 a 48 ± 12 mmHg (p < 0,05). La proteinuria se redujo de 2,74 ± 1,6 a 0,9 ± 1 g/24 h (p < 0,001), representando un descenso promedio del 67,1 %. Según los polimorfismos del SRA, la respuesta antiproteinúrica fue: polimorfismo del gen del angiotensiógeno: genotipo TT: 76,8 %; genotipo MM: 67,3 %; genotipo MT: 65,8 %, significativamente mayor (p < 0,05) para genotipo TT respecto de MM y MT. Polimorfismo del gen de la ECA: genotipo DD: 71,4 %; genotipo ID: 60,6 %, genotipo II: 34,8 %, significativamente mayor (p < 0,05) para genotipo DD respecto a ID e II, y asimismo (p < 0,05) para el genotipo ID respecto a II. Polimorfismo del gen del rAT1: genotipo AC: 85,2 %; genotipo CC: 73,7 %; genotipo AA: 62,7 %; significativamente mayor para el genotipo AC (p < 0,05) respecto a AA y CC. Las diferencias entre la proteinuria inicial y final del período de seguimiento fueron significativas (p < 0,01) para las asociaciones genotípicas: DD/AA, DD/MT, DD/MM, DD/TT y DD/AC, si bien la asociación con mayor efecto antiproteinúrico fue DD/AC (89,9 %, p < 0,05 %). Conclusiones: La administración de dosis altas de olmesartán en pacientes con nefropatía proteinúrica no diabética comporta reducciones significativas en la proteinuria. Este descenso fue independiente del control tensional y de otros factores de confusión. Los polimorfismos del SRA pueden modular la respuesta antiproteinúrica al tratamiento con ARAII.
Objective: To assess the antiproteinuric response to multifactorial treatment based on high doses of angiotensin II receptor antagonists (ARBs) (olmesartan) in patients with non-diabetic proteinuric nephropathies, according to three renin-angiotensin system (RAS) polymorphisms: insertion/deletion of the angiotensin converting enzyme (ACE) gene, the angiotensinogen gene M235T and the angiotensin II type 1 receptor (AT1R) A1166C. Material and method: We studied 53 patients with non-diabetic proteinuric nephropathy with a mean progression time of 84.4±15 months. 41 were males (77.4%); mean age 49.7±3 years, body mass index 30±6kg/m2. All received olmesartan (40mg/12h) associated with a mean of 2.4±1.6 antihypertensive drugs for a median period of 13 months (interquartile range 7-25 months). Results: Systolic blood pressure (BP) decreased from 145±14mmHg to 128±14mmHg (P<.001) and diastolic BP from 85±11mmHg to 79±7mmHg (P<.01). Pulse pressure decreased from 53.5±14mmHg to 48±12mmHg (P<.05). Proteinuria decreased from 2.74±1.6g/24h to 0.9±1g/24h (P<.001), representing a mean decrease of 67.1%. According to RAS polymorphisms, antiproteinuric response was: angiotensinogen gene polymorphism: genotype TT: 76.8%; genotype MM: 67.3%; genotype MT: 65.8%, significantly higher (P<.05) for genotype TT compared to genotypes MM and MT. Polymorphism of the ACE gene: genotype DD: 71.4%; genotype ID: 60.6%, genotype II: 34.8%, significantly higher (P<.05) for genotype DD compared to genotypes ID and II, and also (P<.05) for genotype ID compared to II. AT1R gene polymorphism: genotype AC: 85.2%; genotype CC: 73.7%; genotype AA: 62.7%; significantly higher for genotype AC (P<.05) compared to genotypes AA and CC. The differences between initial and final proteinuria for the follow-up period were significant (P<.01) for genotypic associations DD/AA, DD/MT, DD/MM, DD/TT and DD/AC, although the association with the highest antiproteinuric effect was DD/AC (89.9%, P<.05%). Conclusions: Administering high doses of olmesartan in patients with non-diabetic proteinuric nephropathy results in significant reductions in proteinuria. This decrease was independent of blood pressure control and other confounding factors. RAS polymorphisms may modulate the antiproteinuric response to treatment with ARBs.
INTRODUCTION
Chronic kidney disease (CKD) is a very prevalent entity in the general population1 and it is associated with a significant increase in cardiovascular risk.2,3 In addition, proteinuria is associated with a progression of CKD and constitutes a first degree cardiovascular risk factor. Therefore, the treatment of these patients requires a multifactorial approach aimed at reducing overall cardiovascular risk and the progression to end-stage renal failure (ESRF).4
Angiotensin II receptor antagonist (AIIRA) drugs at high doses have shown their excellent tolerability and effectiveness for reducing proteinuria.5-7 Among the many factors involved in the regulation of the pharmacological response to renin-angiotensin-aldosterone system (RAAS) inhibitors, the influence of different SRA8,9 genotypes must be highlighted.
Several polymorphisms of the genes encoding RAS have been described, the most studied are angiotensin-converting enzyme (ACE) gene, angiotensinogen (AGT) gene, and type I angiotensin receptor (AT1R) gene. ACE gene insertion/deletion polymorphism gives rise to increased plasma ACE activity in patients with the deletion, and associations have been found with increased risk of ESRF, with regards to early ages, patients with polycystic kidney disease10 and IgA11,12 nephropathy, and also with lower kidney-transplant survival,13 and plays a significant role in diabetic kidney hyperfiltration, initiation of diabetic nephropathy and response to treatment.14
Angiotensinogen M235T polymorphism associates T allele with essential hypertension (HT), the development of CKD and ESCRF.15,16 The A1166C polymorphism has been associated with renal and cardiovascular diseases. The C allele is associated with an increased response to angiotensin II,17 progressive deterioration of renal function in CKD patients, higher blood pressure (BP) and left ventricular hypertrophy in hypertensive patients.18 In addition, compared to AT1R polymorphism, there seems to be an association with increased angiotensin II activity both intrarenal and systemic.19
In addition, there are studies on the interaction between different SRA polymorphisms and the progression of cardiovascular and renal disease. These interactions were observed between ACE I/D and AGT M235T polymorphisms, and between ACE I/D and AT1R polymorphisms.20-22
That said, there is little information on the influence of RAS polymorphisms on antiproteinuric effect of pharmacological blockade of RAS in patients with non-diabetic proteinuric nephropathies.
As a result, the aim of this study was to assess, in the long-term, the antiproteinuric response to high doses of AIIRAs (olmesartan) in patients with non-diabetic proteinuric nephropathies according to different RAS polymorphisms.
PATIENTS AND METHOD
This is an observational, open, study with random selection of patients with non-diabetic proteinuric nephropathies treated in our nephrology external consultation offices, over a period of 24 months.
On their first visit all patients were submitted to the standardised diagnostic protocol for chronic kidney disease, which also includes, in addition to medical record and physical examination, a series of laboratory tests, electrocardiogram and abdominal ultrasound. In addition, a blood sample was also taken to determine RAS polymorphisms. Once these results were studied, we performed a renal biopsy to establish the histopathological diagnosis of nephropathy.
After obtaining informed consent, we included 53 patients in this study, 41 were males (77.4%); average age 49.7 ± 3 years, diagnosed by a renal biopsy study with: IgA glomerulonephritis 16 (30.2%), nephroangiosclerosis 12 (22.6%), membranous glomerulonephritis 12 (22.6%), focal segmental glomerulosclerosis 9 (17%) and other nephropathies 4 (7.6%). The 12 patients diagnosed with nephroangiosclerosis had proteinuria >2.5 g/24 hours.
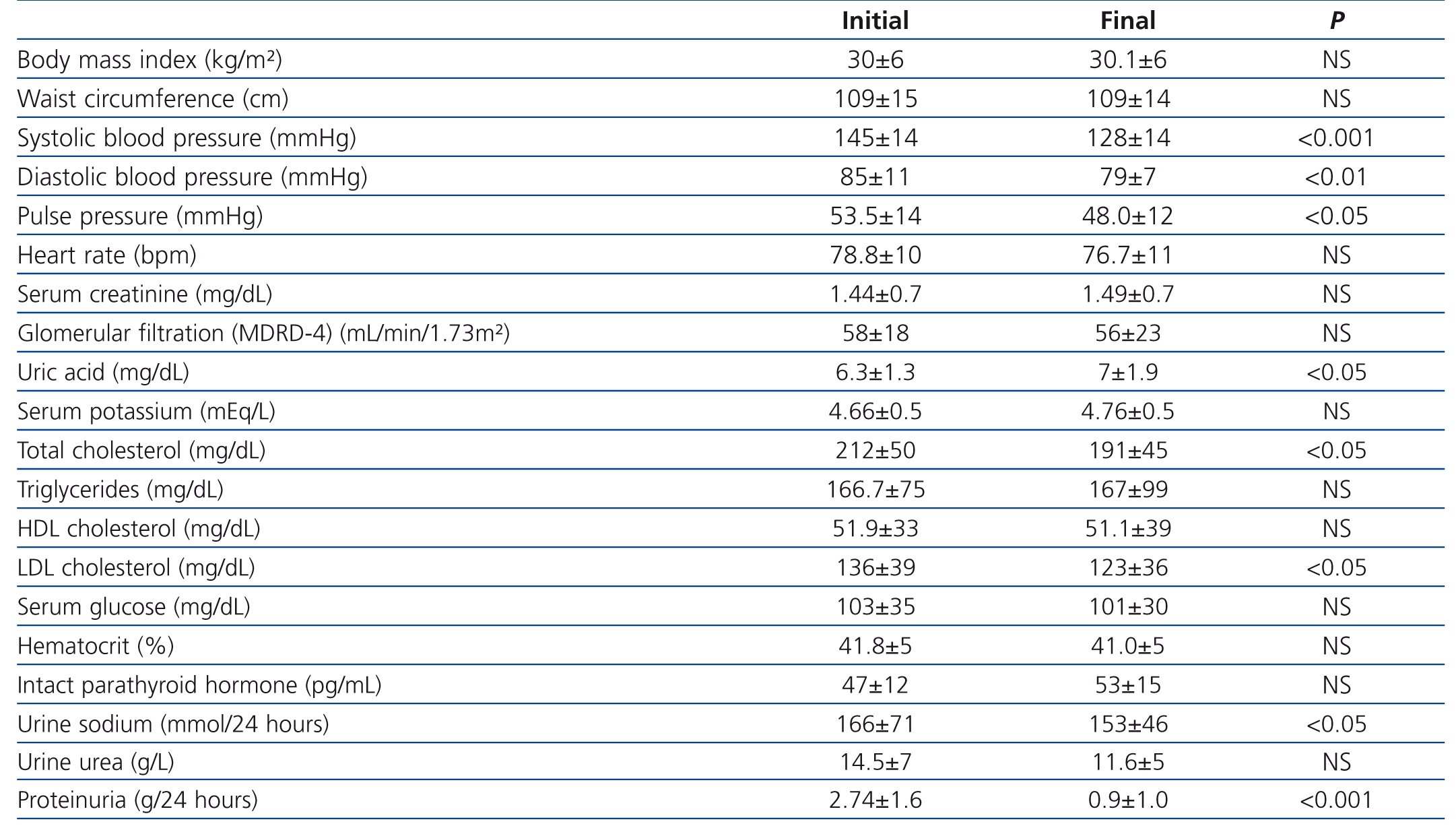
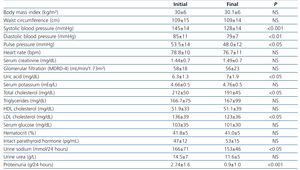
Table 1 shows demographic and anthropometrics characteristics, blood pressure and laboratory values at time of recruitment. Baseline blood pressure values (mean of second and third readings on consultation) of the patients that, with an average progression of 84.4 ± 15 months, were not under treatment with RAS blockers (15-28.3%), or if they were, underwent a 4-week clearance period without RAS blockers. Among the nephropathy-associated cardiovascular risk factors, 46 patients (86.8 %) were hypertensive (evolution from diagnosis: 105±3 months), 36 patients (67.9%) showed mixed dyslipidaemia and 28 (52.8 %) were hypercholesterolemic. Active smokers 16 (30.2 %), whereas 39 (73.6%) had overall obesity, and 41 (77.4%) central obesity (waist circumference ≥102 cm in men and ≥82 cm in women). Given the presence of proteinuria and associated comorbidities, all patients were classified as of very high vascular risk.23,24
RAS polymorphisms were performed by Taqman method and real-time polymerase chain reaction8,10 in the Department of Molecular Biology of the School of Medicine of Malaga, after positive evaluation of the study protocol by the ethics committee.
All patients underwent follow up every 6 months, and the median time follow-up was 13 months (inter-quartile range 7-25 months) until final evaluation. At all follow-up visits, clinical evaluation was assessed (patient's general subjective status, anthropometric and BP measurements) and patient analytical controls were performed, including renal function tests, 24-hour proteinuria, ionogram and metabolic biochemical profile. For the purpose of this study, changes in clinical and laboratory parameters at end of follow-up were compared with baseline values.
Treatment
All patients were treated multifactorially according to the K/DOQI 2006 guidelines recommendations with the aim of achieving proposed therapeutic targets posed to control proteinuria and blood pressure. In general, patients received a hypocaloric diet, low in salt (4-6 g/day), saturated fats or cholesterol, with moderate protein restriction (0.6-0.8 g/kg/bw/day). Thirty-eight patients (71.7%) were treated with statins and 19 (35.8%) were treated with antiplatelet agents. All were given olmesartan-medoxomil at a fixed dose of 40mg morning and evening (18-20 hours), based on the fact that this drug has demonstrated a better 24-hour antihypertensive efficacy profile in comparison with other drugs of the same therapeutic group, providing greater angiotensin II blockade.25,26
For optimal BP control, all patients received additional antihypertensive treatment and the target value was less than 130/80 mmHg. The average number of antihypertensive drugs was 2.4±1.6 per patient, with a predominance of the combined use of loop diuretics (98.2%) and without considering the use of aldosterone. Calcium antagonists were the second most used drug (32%), followed by alpha-blockers (30.2%). Less frequently used were beta-blockers (13.2%) and alfabetablockers (9.4%). No patients abandoned the study during the follow up period due to relevant treatment-related clinical or biochemical side effects.
Data statistical analysis
The results obtained in the different groups of samples are expressed as mean ± standard deviation or median and interquartile range. Differences between qualitative variables were determined using Fisher’s or Χ2 test, as appropriate. The differences between initial and final quantitative parameters were analysed by Student's t test for paired data or Wilcoxon’s test if they did not have a normal distribution. In addition, covariance analysis (ANCOVA) was also performed, using the general linear model for baseline variables and late confusion that could influence initial or final proteinuria, using as fixed factors the polymorphism genotypes studied. We used Pearson's r correlation to determine the relationship between two quantitative variables or Spearman’s correlation to analyse the relationship between a quantitative variable and ordinal one. Values of P<.05 were considered to be statistically significant. Statistical software SPSS for windows version 10 was used.
RESULTS
Throughout the study, no significant differences in mean body mass index (BMI), waist circumference and heart rate were detected (Table 1). However, systolic BP, diastolic BP and pulse pressure decreased significantly at the end of follow-up.
There was an average decrease of proteinuria of 1.64±1.7 g/24 hours, equivalent to a percentage decrease of 67.1%. However, no significant changes were seen between initial and final values of serum creatinine, estimated glomerular filtration according to MDRD-4 (Modification of Diet in Renal Disease), serum potassium, and only uric acid values increased significantly at the end of the study.
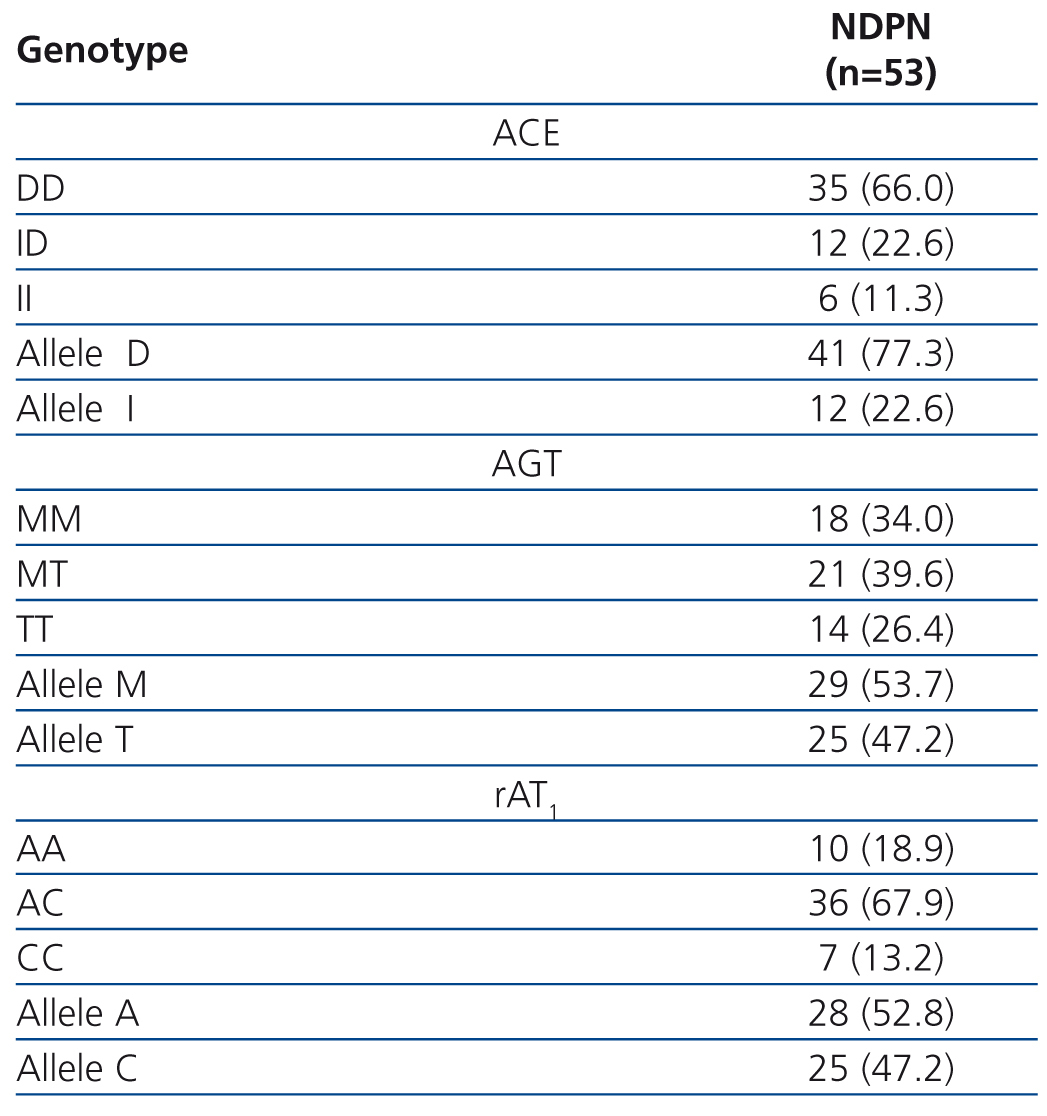
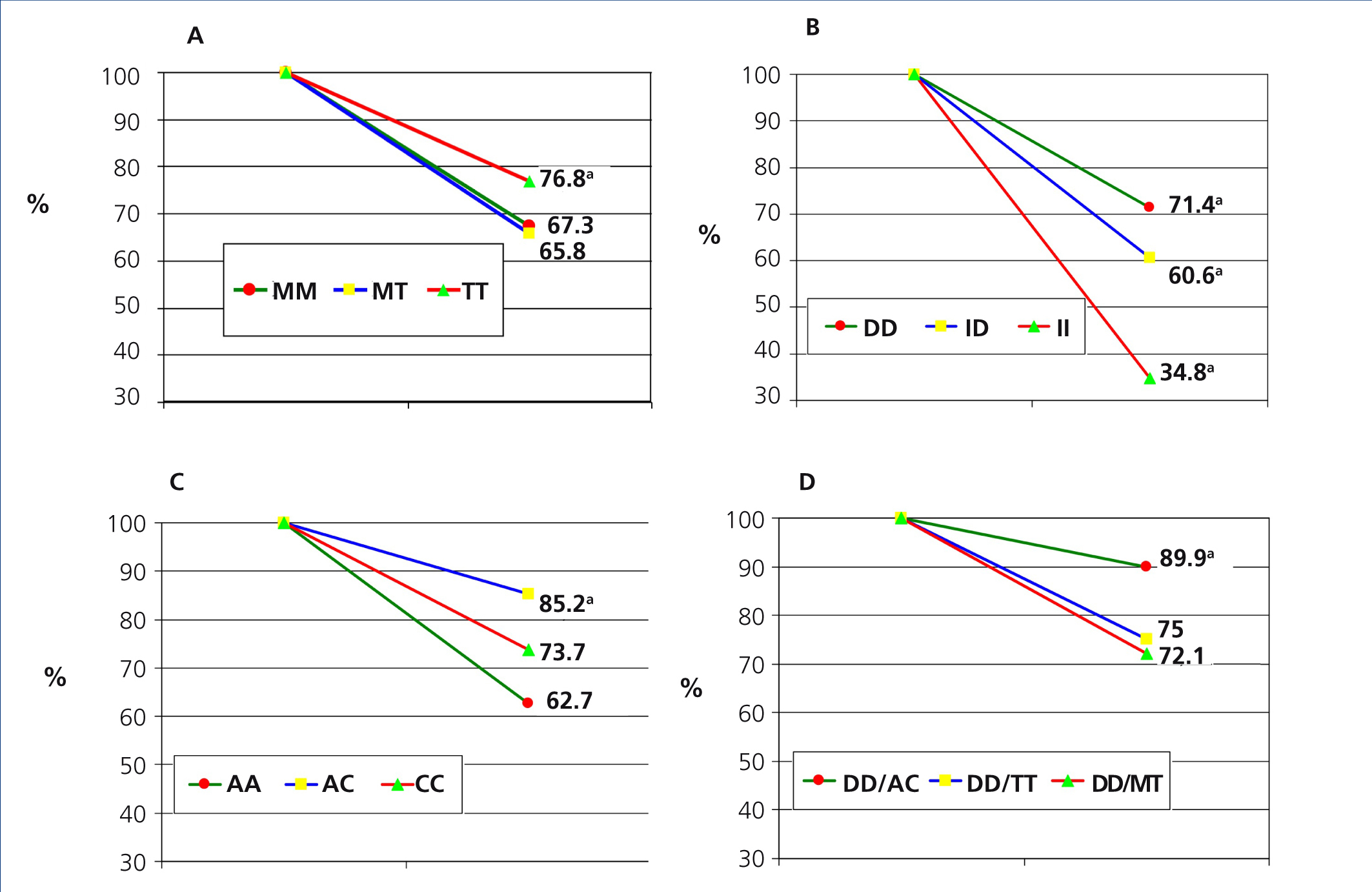
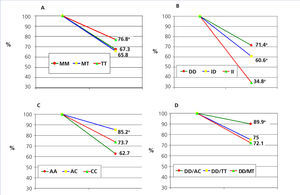
Table 2 describes the frequency distribution of RAS genotypes of our patients. The percentage decreases in proteinuria according to different RAS genotypes are collected in Figure 1. The independent contribution of different polymorphisms in the antiproteinuric response was as follows: 1) Angiotensinogen polymorphism: patients with the TT genotype reduced proteinuria by 76% compared with MM genotypes (67.3%) and MT (65,8%), with significant differences (P<.005). 2) ACE polymorphism: patients with DD genotype reduced proteinuria to a greater extent (71.4%) compared to genotype ID (60.6%) and genotype II (34.8%) patients, both with P<.05. 3) For AT1R polymorphisms, the decline of proteinuria was 85.2% in those with genotype AC, compared to 73.7 % in patients with CC genotype and 62.7 % for AA genotype, this difference was significative (P<.001). In all cases, there were no significant difference in final BMI and PA according to the polymorphisms studied.
Finally, the analysis of the associations of the different genotypes (Figure 1 D) showed that the three associations with greater antiproteinuric synergistic effect were combined genotypes DD/BC (89.9%) (P<.05 %) compared to DD/TT (75.0 %) and DD/MT (72.1%).
No correlation was seen between baseline and final proteinuria levels related to different clinical parameters that could influence proteinuria, such as age, sex, smoking, BMI, systolic and diastolic BP, or time of evolution of the nephropathy. During covariance analysis, proteinuria levels were associated with RAS polymorphisms studied, adjusting for other confounding factors such as BP, renal function and BMI.
DISCUSSION
The major finding of our study was that the use of high doses of olmesartan significantly reduces proteinuria in patients with non-diabetic nephropathy and this effect could be modulated by polymorphisms of several RAS genes, regardless of blood pressure control and other confounding factors. Indeed, although BP significantly decreased in all patients according to previous studies,27-29 proteinuria levels were not associated with BP changes, as noted in the covariance analysis. In addition, other potential confounding factors, such as BMI or renal function, had no significant impact on proteinuria levels.
One objective of the study was to obtain, within a multifactorial therapeutic approach, as recommended in K/DOQI30 and European24 guidelines, optimisation of RAS blockage in patients in order to reduce proteinuria to a greater extent than standard doses of AIIRAs, in line with results seen by other authors.31 Consequently, we began treatment with high doses of olmesartan (40mg twice a day), and obtained, regardless of the degree of BP control, an excellent antiproteinuric response (decrease of 67.1% over baseline proteinuria). We did not rule out that the decrease in 24-hour natriuresis (P<.05), attributable to lower sodium intake, may have increased RAS blockage in our patients, by modulating the response to SRA32 blockers and thereby enhancing antiproteinuric response.
Unlike the kaliemia increases observed when treating CKD patients with high doses of ACE inhibitors (ACEI) or the combination AIIRA/ACEI33, the use of high doses of AIIRAs produced no significant changes in potassium levels, in spite f the fact that most patients had stage 3 CKD. Although the main use of loop diuretics in our patients could have contributed to maintaining a low level of potassium, other well known reasons could explain why AIIRAs cause less increase than ACEI in cases of kalemia. In addition, as shown by different studies in the medical literature,34 we cannot exclude that the significant improvement in lipid profile attributed to the widespread use of statins (71.7% of patients) could also have contributed to a decline in proteinuria.
Regarding AT1R genotypes, we observed an AA genotype frecuency of 18.9%, significantly lower (P<.001), and an AC genotype frequency of 67.9%, statistically greater (P<.001). The low frequency of subjects with genotype AA, and the high frequency of genotype AC genotype, are due to the fact that our sample is relatively small, since in a previous study with 745 patients with CRD22 we found a frequency of 46.5% for genotype AA and 43.2% for genotype AC. Similar percentages are given in Fabris’ study35 on renal failure patients.
When we analysed the antiproteinuric response to ARBs in our sample according to different genotypes, we found that angiotensinogen genotypes seem to participate equally in the antiproteinuric response to ARBs, although, in patients with TT genotype with greater activation of the system, the percentage decline of proteinuria is somewhat higher than in patients with MM and MT genotypes (P<.05).
Our results are consistent with other studies that have shown a benefit with pharmacological RAS blockade in patients with DD genotype.36 Antiproteinuric response in DD patients was significantly higher (P<.05) than in those with ID genotype and especially ACE II. Therefore, our results could be related to increased plasma ACE activity and/or increased urinary albumin excretion in these patients.37 In other words, a greater benefit in antiproteinuric response would be expected in those patients with higher levels of ACE. Along this same topic, the Syrjänen et al.38 study shows that individuals with ACE DD genotype have higher antiproteinuric response in patients with IgA nephropathy. In addition, this fact has been observed in patients with diabetic nephropathy, where the response to treatment with ACE inhibitors in diabetics with ACE DD genotype was greater.39,40
In the analysis of response to AT1R genotypes, we noted that those subjects with C allele exhibit a significantly higher response to intense pharmacological blockage of AT1R.
In the analysis of genotype associations with greater antiproteinuric effect, we saw that DD/AC subjects have a greater antiproteinuric response (89.9%), which we interpret as a consequence of increased RAS activation given the higher enzymatic activity of DD genotypes that generate greater amounts of angiotensin II, which is a positive activator of its own receptors,41 considering that the C allele shows an increase of transcription and therefore, of AT1 receptor density.
In Contreras’ study,12 we observed that patients with polycystic renal disease and alleles D, C and G were those with greatest increase in proteinuria and CKD progression. Moreover, the results of our study indicate that patients with D, C and T alleles are those that best respond to RAS pharmacological blockage with an angiotensin II AT1 blockage.
Fabris et al.35 evaluated the possible relationship between RAS genetic polymorphisms and the development of CRF in patients with essential hypertension and renal dysfunction compared to those with no CRF. The study showed a greater association with the AGT T allele and ACE D allele and a possible positive interaction between essential hypertension and development of CRF in AGT-TT/ AT1R-AC y ECA-DD/ AT1R-CC associations. The data of this study are largely consistent with ours, since patients with D and G alleles are those which, in this case, progress more frequently to CRF.
The main limitation of our study was the sample size, so it would be wise to consider a larger cohort of patients or conduct a clinical trial in patients with CKD and non-diabetic proteinuria to elucidate possible differences or interactions between different RAS genotypes.
In conclusion, high-dose olmesartan decreases proteinuria in patients with non-diabetic proteinuric nephropathies and this effect appears to be mediated by unfavourable RAS polymorphism genotypes, regardless of blood pressure control and other confounding factors. This may help to determine antiproteinuric treatment based on RAS genotypes in these patients.
Acknowledgements
This study was financed in part by the Spanish Ministry of Science and Innovation (Carlos III Health Institute, FIS PI10/01020), REDINREN RD12/0021/0015 and by the Andalusian Regional Ministry of Health (PI-0590/2012).
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Anthropometric, blood pressure and laboratory data at baseline and end of study
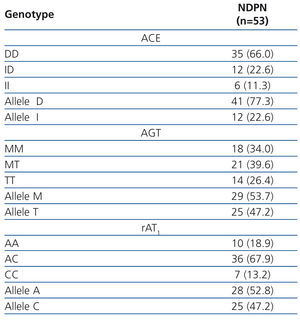
Table 2. Cases and frequency distribution (%) of different renin-angiotensin system genotypes in patients with non-proteinuric diabetic nephropathy
Figure 1. Lowering of proteinuria depending on renin-angiotensin genotypes