Introducción: La candidosis bucal (CB) es una infección oportunista frecuente en el paciente inmunocomprometido y algunas veces es importante conocer la especie para el tratamiento. Objetivo: Determinar la prevalencia de distintas especies de Candida colonizando o infectando la mucosa bucal (MB) de pacientes diabéticos (DM) y no diabéticos (no DM) con enfermedad renal crónica, comparando ambos grupos y explorando algunos posibles factores de riesgo. Metodología: Se examinó a 56 pacientes DM y 80 no DM con diálisis crónica. Se tomaron muestras de la MB y se sembraron en agar placas dextrosa Sabouraud. La especie se identificó con galerías API®. La CB se confirmó con citología exfoliativa. Las asociaciones se investigaron con χ2, Prueva exacta (PE) de Fisher y regresión logística múltiple. Resultados: La prevalencia de Candida fue del 43,4 %: 53,6 % DM y 36,2 % no DM (p = 0,045). Las especies fueron C. albicans 74,6 %, C. glabrata 22,0 %, C. tropicalis 15,2 %, C. parapsilosis 3,4 %, C. kefyr 3,4 % y C. famata 1,7 % sin diferencia entre grupos. Los DM tuvieron mayor frecuencia de xerostomía (p = 0,002), flujo salival bajo (p = 0,008) y albúmina sérica más baja (p = 0,018). Tuvieron CB 16,9 %, 23,2 % DM frente a 12,5 % no DM (p = 0,101). Se asociaron a presencia de Candida en la MB: uso de prótesis (odds ratio [OR] 25,6, límite de confianza [LC] 95 % 2,5 a 253, p = 0,001), xerostomía (OR 9,6, LC 95 % 2,4 a 38,1, p = 0,001) y bajos valores de albúmina sérica (OR 0,41, LC 95 % 0,22 a 0,98, p = 0,044). Conclusiones: La presencia de Candida sp. en la MB se asoció a prótesis dental, xerostomía y albúmina sérica baja.
Introduction: Oral candidiasis (OC) is a common opportunistic infection in immunocompromised patients. Species identification is sometimes important for treatment. Objective: to determine the prevalence of different Candida species colonising or infecting the oral mucosa (OM) of diabetic (DM) and non-diabetic (non-DM) chronic kidney disease patients, comparing both groups and exploring potential risk factors. Methods: 56 DM and 80 non-DM patients on chronic dialysis were examined. OM swabs were cultured on Sabouraud dextrose agar plates. Candida species were identified with API® galleries. OC was confirmed by exfoliative cytology. Statistical associations were analysed using χ2, Fisher’s exact test (ET), and multiple logistic regression. Results: Candida prevalence was 43.4%: 53.6% DM and 36.3% non-DM, (p=.045). The species identified were C. albicans 74.6%, C. glabrata 22.0%, C. tropicalis 15.2%, C. parapsilosis 3.4 %, C. kefyr 3.4% and C. famata 1.7% without difference between groups. DM patients had a higher xerostomia prevalence (p=.002) and lower salivary flow (p=.008) and lower serum albumin (p=.018). 16.9% of patients had OC, 23.2% DM compared with 12.5% non-DM, (p=.101). The following were associated with the presence of Candida in the OM: the use of dental prostheses (odds ratio [OR] 25.6, 95% confidence interval [CI] 2.5 to 253, P=.001), xerostomia (OR 9.6, 95% CI 2.4 to 38.1, P=.001) and low serum albumin values (OR 0.41, 95% CI 0.22 to 0.98, P=.044). Conclusions: The presence of Candida sp. in the OM was associated with dental prostheses, xerostomia and low serum albumin.
INTRODUCTION
Fungi of the genus Candida are a group of ubiquitous yeasts with opportunistic behaviour, that are part of the microbiota of the oral mucosa (OM) and the female gastrointestinal and genital tract, which it may colonise in a third of the general population.1,2 Colonisation risk factors depend on the fungus’ characteristics and in particular, its adhesion to epithelial cells, as well as local OM factors and the host’s systemic condition, for example, diabetes mellitus or immunosuppression of various causes.1,3Candida albicans is the most prevalent species in colonisation of the OM in healthy and immunocompromised individuals; nevertheless, C. parapsilosis, C. tropicalis, C. glabrata, C. krusei, C. guilliermondii and C. dubliniensis have also been reported.1,2,4 Colonisation of the OM by Candida sp. is a risk factor for progression to oral candidiasis (OC), both in immunocompetent and immunocompromised individuals.1,5 OC is due to the proliferation of fungi on the mucosa surface and it may come from the microbiota of the same patient in a carrier state.4 Its growth is explained by fungal mechanisms and the host’s defences.3,6 The coexistence of different Candida species in an infection makes it more persistent and difficult to treat.7,8
In Mexico there is no chronic kidney disease (CKD) patient registry. Several authors have reported that diabetes is the most common cause of CKD in adults, its incidence is increasing and it is a public health problem.9-11 Chronic kidney disease patients on dialysis have a high frequency of anaemia, malnutrition and multifactorial immunological deterioration, and as such, they are considered to be immunosuppressed.12,13
In the oral context, it has been reported that these patients have decreased salivary flow (SF), xerostomia and mucosal atrophy,14,15 conditions that are conducive to OC development both in diabetic (DM) patients without complications and in DM patients16 with CKD on dialysis.14,15,17 Although prevalence of colonisation and OC has been reported in different groups of immunocompromised patients, few studies have been conducted on dialysis patients. The purpose of this study was to determine the prevalence of different Candida species that colonise or infect the OM of DM patients with CKD on dialysis and compare them with non-diabetic (non-DM) patients with CKD on dialysis and analyse some potential risk factors.
MATERIAL AND METHOD
Study population
A cross-sectional study in which we examined DM and non-DM CKD patients of the Haemodialysis Unit at the Hospital General de Zona No. 50 from the Instituto Mexicano del Seguro Social in San Luis Potosí, Mexico. The study had previously been approved by the hospital’s research and ethics committees. We collected demographic data, kidney disease data, the progression time of diabetes mellitus (where applicable) and clinical laboratory data from the patients’ clinical records. All patients were requested their informed consent for us to take oral samples. We excluded patients with unstable clinical conditions who were not suitable for oral examination and/or SF measurement. We excluded cases when the culture became contaminated and we were unable to contact the patient in order to obtain a new sample. The oral examination was carried out by a specialist in Oral Pathologies and Medicine. The culture sample was taken in the morning and at least two hours after eating food or intaking liquids and the patients were asked about their use of dental prostheses (DP). Before taking samples for the microbiological culture, we measured SF using Schirmer’s test, which consists of measuring accumulated saliva in the floor of the mouth for five minutes using Whatman strips (millimetrically graded filter paper). We considered the SF to have decreased whenever the reading was ≤2.0cm.18 The clinical criteria for the diagnosis of OC were those of Holmstrup and Axéll (erythematous or pseudomembranous candidiasis or prosthesis-associated candidiasis).19 When a lesion suggestive of OC was identified in the oral examination, we performed an exfoliative cytology, which was stained with Periodic acid–Schiff to confirm the infection. The patient was considered to have an infection whenever there was a clinical lesion and the exfoliative cytology was positive.
Microbiological methods and species identification
For fungal identification, we took samples of the whole OM of each patient with sterile swabs, which were coded for their subsequent inoculation on Sabouraud dextrose agar plates with chloramphenicol and on Biggy agar plates. Candida was characterised by various phenotypic methods, including cultures, germ tube formation in human serum and biochemical profiles with API® galleries. We considered as colonised (healthy carrier) asymptomatic patients without oral lesions and with mucosa swab culture positive for Candida sp. The plates were incubated at 36.5±0.5°C, with growth readings being performed after 24, 48 and 72 hours. With the Biggy agar culture, we carried out a presumptive identification of the Candida species, in accordance with the colorimetric characteristics; furthermore, the use of this means allowed us to identify the presence of two or more Candida species in the same sample. Positive cultures in one or more species were re-inoculated and purified in Sabouraud glucose agar plates and were incubated at 36°C (±1ºC) for two days. The Candida species were identified by the carbohydrate assimilation system API 20 C AUX (BioMerieux, Lyon, France).
Statistical analysis
We performed a descriptive analysis of the demographic and clinical variables, age, sex, body mass index, causes of chronic renal failure (CRF) and clinical laboratory data. Comparisons were made with the Student’s t-test and χ2. The presence or absence of Candida in the OM was graded on an ordinal scale of three levels: 1) absent, 2) carrier and 3) present with candidiasis. Potential risk factors were investigated using multiple logistic regression analysis, with candidiasis as the dependent variable and the independent variables being sex, genus, CKD data, laboratory data and the use of DP. We used the Epi Info version 3.4.3 software and considered a P-value of <.05 to be statistically significant.
RESULTS
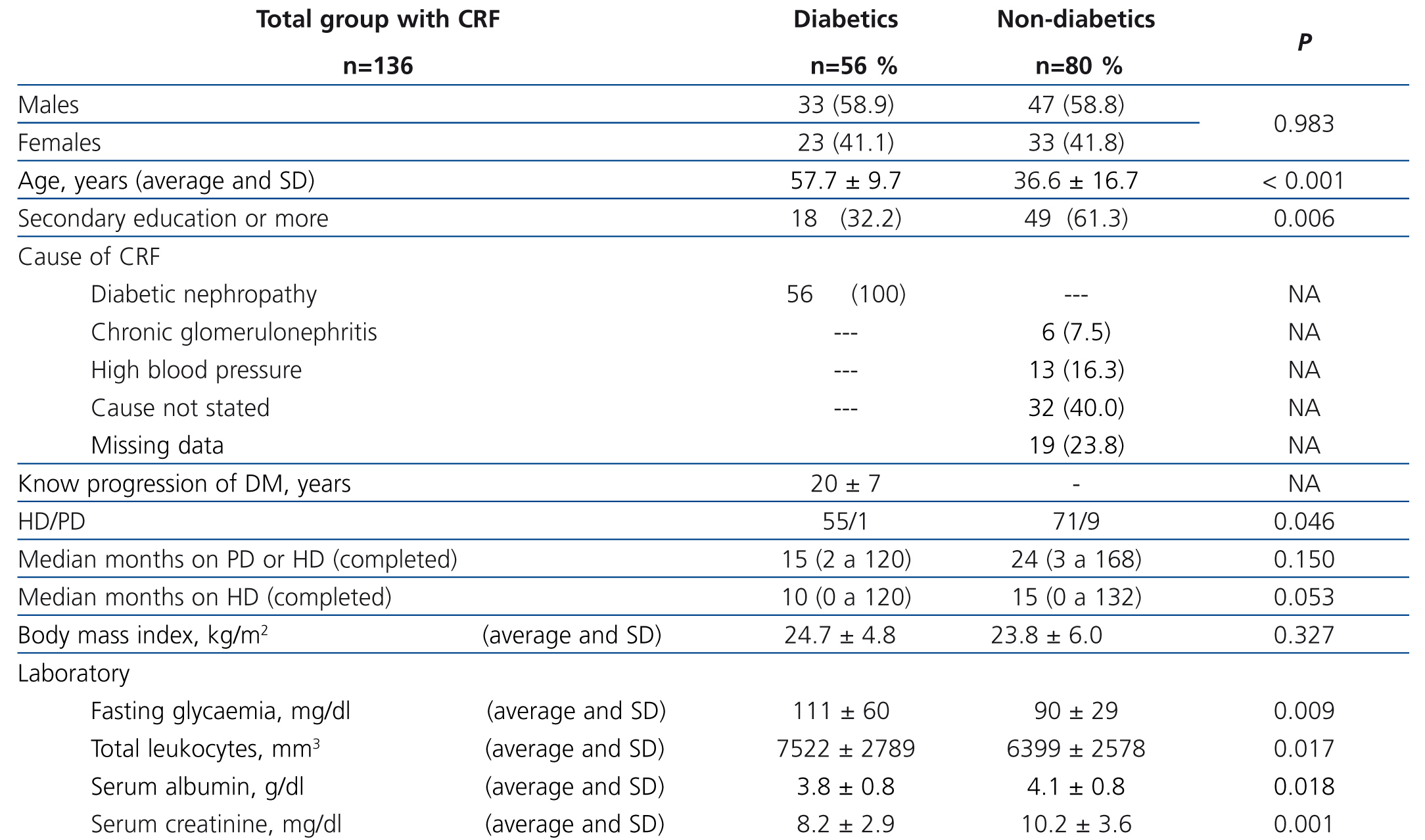
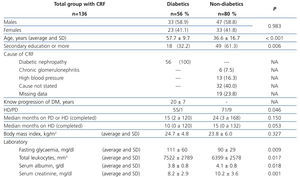
We studied a total of 136 patients (56 DM and 80 non-DM), 80 males and 56 females, with an average age of 45.3±17.6 (14-86) years old. One hundred and twenty-six were on chronic haemodialysis (HD) and 10 on peritoneal dialysis, with a median treatment time of 24 (2 to 168) months. Table 1 shows a comparative analysis of demographic and clinical characteristics and laboratory results for both groups. Twenty-four patients (17.6%) reported a history of smoking, with there being no difference between groups.
Oral conditions
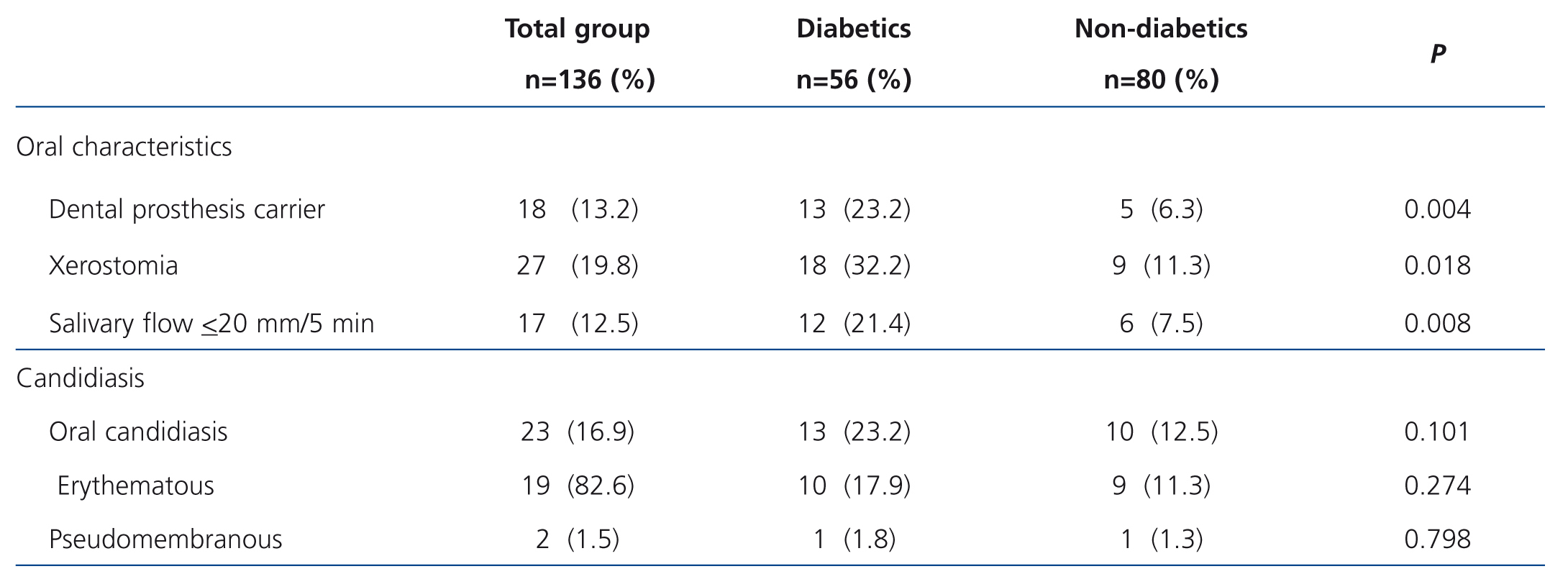
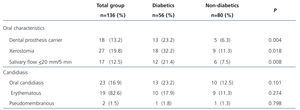
Table 2 shows that DM patients more commonly used DP (P=.004), had higher xerostomia (P=.018) and lower SF (p=.008). Eighteen patients (13.2%) used DP.
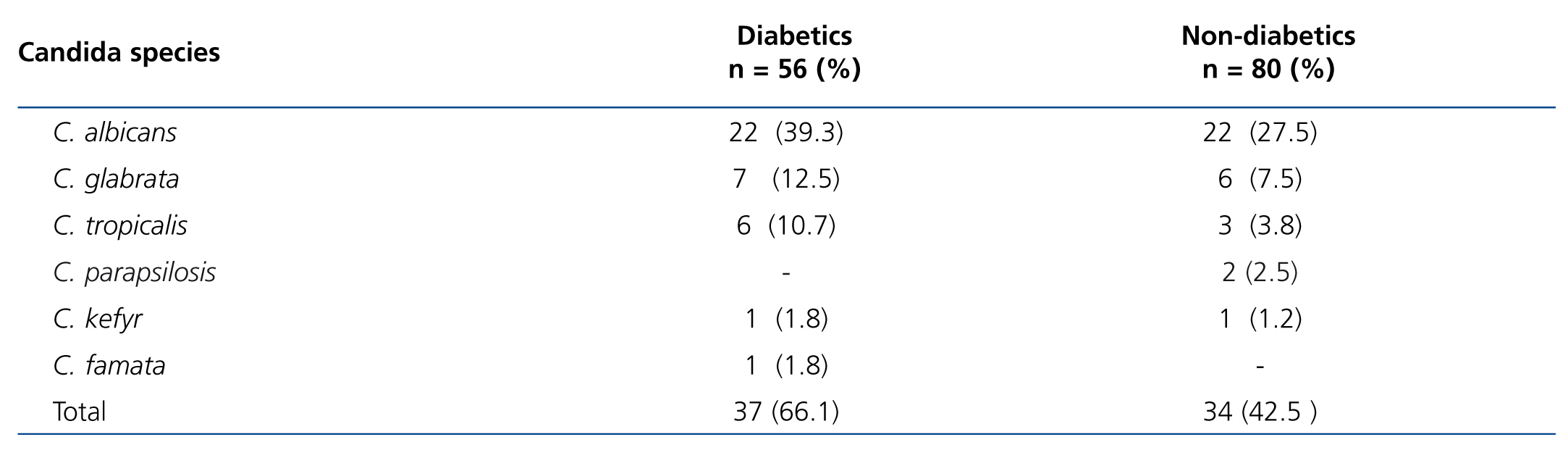
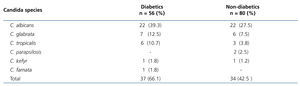
The prevalence of Candida in OM was 43.4% (59/136): 53.6% (30/56) in DM and 36.2% (29/80) in non-DM (P=.045). As regards Candida species, we did not observe a difference between DM and non-DM. Table 3 displays the frequency by species of the total isolated Candida and cases with two or more species in the same patient. The frequencies per species of the total positive isolated Candida (71) were: C. albicans 44 (74.6%), C. glabrata 13 (22.0%), C.tropicalis 9 (15.2%), C. parapsilosis 2 (3.4%), C. kefyr 2 (3.4%) and C. famata 1 (1.7%). Eleven patients (8.1%) had more than one Candida species; one patient had C. albicans/C. glabrata/C. tropicalis, 5 patients had C. albicans/C. glabrata, 3 patients had C. albicans/C. tropicalis, one had C. glabrata/C. tropicalis and one had C. glabrata/C. parapsilosis.
We observed OC in 16.9% (23/136) of the total group, 23.2% (13/56) in DM-CRF patients and 12.5% (10/80) in non-DM patients (P=.101). Of the 18 DP users, 55.6% (10/18) had prosthesis-associated OC, 61.5% (8/13) were DM patients and 40% (2/5) were non-DM patients (P=.608, Fisher’s ET). In relation to the clinical type, 19 patients (82.6%) displayed the erythematous type on the dorsum of the tongue and one patient had erythematous and pseudomembranous candidiasis and DP-associated candidiasis. The Candida species identified in cases with candidiasis were C. albicans 17 (73.9%), C. tropicalis 7 (30.4%), C. glabrata 4 (17.4%) and C. kefyr 1 (4.3%). In the 36 cases without candidiasis, we identified: C. albicans 27 (75.0%), C. glabrata 9 (25.0%), C. parapsilosis 2 (5.6%), C. tropicalis 2 (5.6%), C. kefyr 1 (2.8%) and C. famata 1 (2.8%). We did not observe a difference between groups (those with and without candidiasis) in the prevalence of C. albicans.
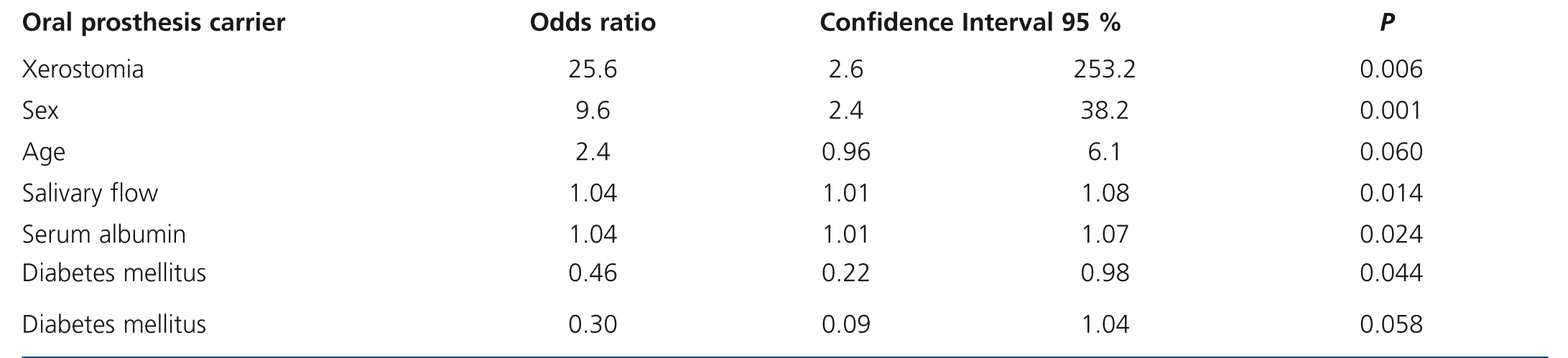
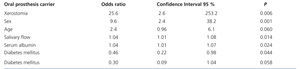
Table 4 shows the final multiple logistic regression analysis, after discarding the variables that showed P values>.50 in previous analyses. We observed an increased relative risk of the presence of Candida in OM (with or without candidiasis) in patients who used DP (p=.006), with xerostomia (p=.001) and with low serum albumin values (p=.044) (the latter was 3.6±0.6g/dl in patients with candidiasis, 3.8±0.8g/dl in carrier patients and 4.1±0.6g/dl in patients not colonised).
DISCUSSION
Our results demonstrated that the presence of Candida in OM, colonising or infecting, was related to the use of DP, xerostomia and low serum albumin.
Colonisation by Candida sp. in the mucosa is a risk factor for superficial infections such as OC, and others of greater significance, i.e. invasive infections, especially in immunocompromised patients.5,17,20 The coexistence of different Candida species in an infection makes it more persistent and difficult to treat.8,21
Colonisation by Candida sp. occurred in 43.4%, a prevalence similar to that reported (39%) in a similar study on patients with CKD.22 Other authors have reported frequencies of 46%23 in DP users with HD, or 51.2% in which the oral microbiota of HD patients was what was measured.24 The frequency observed in this study was lower than that reported in other immunocompromised groups of patients with head and neck neoplasms 56.8%,25 with solid organ transplants 57%,8 with the human immunodeficiency virus (HIV/AIDS) 66.7%,26 at the end of radiotherapy for oral cancer 69%27 and with type 2 diabetes mellitus 77%.21 The foregoing may be explained by the fact that in each group studied, there are major local factors, such as a severe decrease in SF due to radiotherapy, systemic factors such as the use of immunosuppressant drugs or antineoplastic medication that causes xerostomia or immunosuppression due to the underlying disease. CKD patients, despite the known decrease in monocyte functions, systemic deterioration and hyposalivation, are not on average as immunosuppressed as the other patients.12,27-29
Candida albicans was the predominant species (74.6%), without there being a difference between groups (p=.148). This species is the most prevalent both in healthy individuals2 and in those with systemic involvement.7,8,22-25 Its frequencies vary in accordance with the group studied: dialysis patients from 51.7% to 63%,22,23 kidney transplant patients 44%,8,30 patients with cancer in the head and neck 74%,25 patients with infection due to HIV/AIDS from 60.7% to 83.5%,7,26 and in DM patients from 68.9% to 86.5%.21,31
C. glabrata was the second most common Candida species, similar to that observed in another study on chronic dialysis patients.22 In our study, 8 patients simultaneously presented C. glabrata and another species, 4 of them without OC. This mixed colonisation may be a risk factor for developing more severe infections. C. glabrata has been shown to be the second most isolated species after C. albicans in patients with CKD and a renal transplant,8,22 cancer of the head and neck and haematological malignancies.25C. glabrata has been identified as an opportunistic species that in conditions of severe immunosuppression is associated with nosocomial infections, a long dwell time of intravenous catheters, the prophylactic use of antifungal medication (especially fluconazole) and the chronic use of DP.32 This species may cause severe oropharyngeal candidiasis, which is difficult to treat because it is innately less sensitive to fluconazole and itraconazole and results in higher mortality rates when candidaemia occurs.33 -35
OC is the most commonly reported opportunistic fungal infection in CKD patients, with frequencies ranging from 5.7% to 32%.15,17 In this study, OC was present in 17% of patients. The erythematous type was the most common (83%), and xerostomia was a major risk factor. It occurred on the dorsum of the tongue in all cases, with there being no difference between DM and non-DM. This prevalence is similar to that previously reported by us in CKD patients on HD,15 which suggests that the characteristics of our study population are similar in terms of systemic condition. Another factor associated with OC is a lower SF, which results in a loss of the defensive function of saliva.14,15 In this study, DM patients displayed lower SF and a higher frequency of xerostomia, but only xerostomia was associated with OC.
This study confirmed that the use of DP is a risk factor for colonisation and infection by Candida, as shown by the high odds ratio of 25.6 that we observed, since in 83% of patients who used a prostheses, the Candida was isolated. The use of DP is a risk factor for colonisation and/or infection by Candida in the OM and is due to a lack of hygienic care and its use over many years.5,23,24,32 DM patients on HD have a higher frequency of tooth loss, and as such, the use of this dental apparatus is common.36 Furthermore, it was confirmed that the adhesion capacity of the fungus to inert polymers such as acrylic resins, converts the latter into reservoirs, which favours colonisation and/or infection.3,5,32 Other authors found an association between the density of colonisation by the fungus and the progression time of kidney disease, but not of dialysis treatment,23,37 possibly due to the progressive deterioration caused by this disease.
CKD patients on dialysis have a high frequency of nutritional, immunological, and psychological disorders as well disorders from invasive procedures and antimicrobial treatments, which are known to contribute to the presence of a higher number of yeast colonies.12,13,29 Their peritoneal dialysis or HD treatment requires the introduction of central venous or peritoneal catheters, which, as has already been mentioned, are risk factors for invasive infections.38-40 In this regard, the colonisation and/or infection by Candida at different anatomical sites, including the oropharyngeal mucosa, increases the risk of candidaemia, through various mecanisms.17,28,41 The implication of this risk factor for CKD patients on dialysis has been poorly documented.
Furthermore, diabetes mellitus has been described as a risk factor for OM colonisation by Candida.21,31 However, in the multiple logistic regression analysis of this study, it was ruled out as an independent risk factor for both colonisation and OC. One possible explanation is that ckd patients on dialysis may have deteriorated and have other systemic and local risk factors that are relatively more important. Female sex was observed to be only marginally more associated with the presence of Candida in the OM in contrast to that reported by other authors.31,42 Notably, an independent association was found between the presence of Candida colonising and/or infecting the OM and low serum albumin, which supports the notion that hypoalbuminaemia is a reflection of systemic deterioration, with there being a lower capacity to defend against microorganisms. While there have previously been reports on the association between low albumin and the female sex and diabetes in CKD patients on chronic dialysis, particularly peritoneal dialysis13 (where it is interpreted as a negative acute phase reactant),43 our study does not confirm this association, possibly due to the role of albumin as a marker, particularly of nutritional status in HD patients.
CONCLUSIONS
This study was important for identifying the frequency of colonisation and infection by Candida in CKD patients on dialysis, since few studies have been carried out on this group of patients, which in Mexico represents high rates of morbidity and mortality. DM patients had lower SF (hyposalivation), higher xerostomia and low serum albumin, with the latter being a risk factor not previously described for the presence of Candida in the OM in these patients. We should be aware of the diversity found in Candida species, including some highly pathogenic species, such as C. glabrata and C. tropicalis, in the treatment of these patients, since some of them have been reported in severe infections related to dialysis catheters.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic and clinical data of diabetic and non-diabetic patients with chronic kidney disease and chronic dialysis
Table 2. Oral characteristics and candidiasis in 136 patients with chronic kidney disease on dialysis
Table 3. Distribution of Candida species in the oral mucosa of diabetic and non-diabetic patients on chronic dialysis
Table 4. Risk factors for the presence of Candida (with or without candidiasis), in diabetic and non-diabetic patients on chronic dialysis