The dialyser is the main element of haemodialysis, where the exchange of substances between the blood and the dialysis fluid (DF), ultrafiltration (UF) and retrofiltration takes place. Haemodialysis and dialysers have markdly changed since the first dialysis in 1924,1 which used tubular dialysers made of collodion and cellulose trinitrate, known as the Haas dialyser. This review focuses on the changes in dialysers over the last two decades, some of which may not seem to be so obvious.
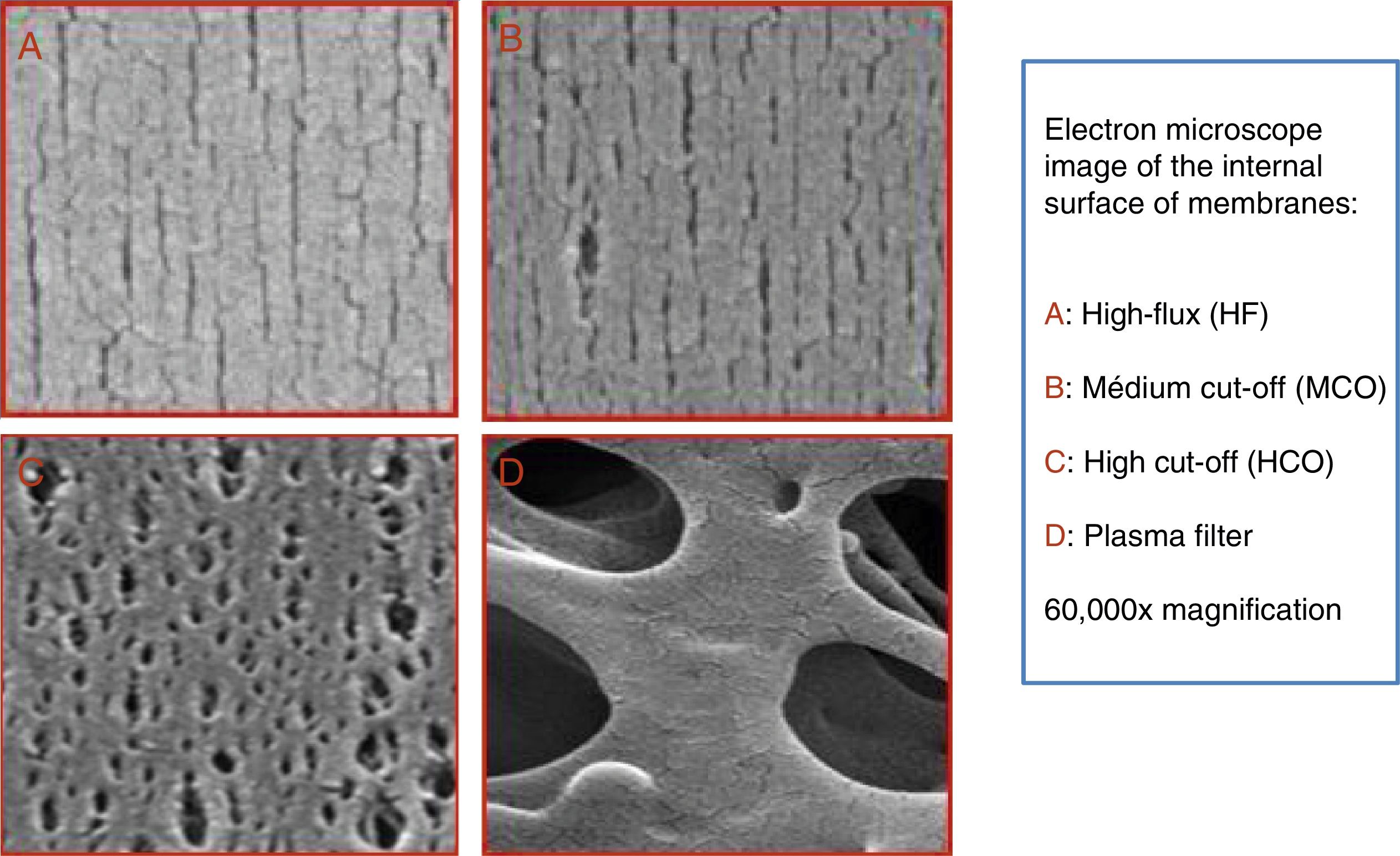
A dialyser is more than a membrane; but it is clear that this is one of its main components. The pores of the dialyser membrane should be of a specific size; should have many pores arranged in a uniform manner. This is achieved with methods that include nanotechnology.2 The formation of a protein layer in the membrane—which is generated as blood comes into contact with the membrane—limits the clearance of molecules by functionally modifying the pores.3 Several of the following specifications rely on the application of nanotechnology.
A dialyser's ultrafiltration coefficient (UFC) is one of its most-valued characteristics. The UFC and the dialyser's design will condition the retrofiltration, which should not generate any concern with the use of ultrapure DF,4 an essential requirement in modern treatment. In fact, it is a way of clear medium- and large-sized molecules without the need of haemodiafiltration (HDF). Modern dialysers require a high UFC, greater than 40ml/mmHg/hr.
The sieving coefficient (SC) should be high for medium-sized molecules, without a significant loss of albumin. In modern haemodialysis, we need clearance markers for medium- and large-sized molecules. The most commonly used is the SC for β2 microglobulin, which should be greater than 0.6 (by the EUDIAL group).5 It is higher than 0.7 in most modern dialysers. The UFC depends on the number of pores, the SC for the β2 microglobulin, its size and uniformity; all this should be accompanied of a minimal clearance of albumin; the SC for albumin should be less than 0.01. The loss of albumin needs to be assessed at the initiation of the session and when the dialyser is under high transmembrane pressures, as it is the case of online haemodiafiltration (OL-HDF). In these conditions, the loss of albumin should be less than 4g per session.6
The DF should be uniformly distributed in the dialyser; there should be no preferential circuits escaping the contact with capillaries. This was improved by including alternating filaments, by undulating fibres and through other methods. Currently it is achieved with very high density of fibres, over 11,000. With these dialysers, the optimum DF flow (Qd) in haemodialysis and OL-HDF is between 400 and 500ml/min7–9; the use of Qd of 700–1000ml/min is not justified.
To achieve clearance of water and small molecular weight solutes it is necessary to retain a certain hydrophilia in the membrane. In synthetic membranes, this is achieved with polyvinylpyrrolidone (PVP). This compound is found in variable quantities in synthetic hydrophobic membranes, ad it is more or less imbued in the membrane. The presence of PVP in the eluate may be related with the allergic reactions that some patients currently develop when exposed to synthetic membranes.10,11 Adverse reactions are the most clear clinical signs of dialyser bioincompatability. These adverse reactions are commonly referred to as Type A and B hypersensitivity reactions. We think that currently it is more useful to define hypersensitivity reactions in accordance with their clinical sign and the cause. There have been basophils degranulation, both specific and non-specific, as reactions to the ethylene oxide [ETO], complement activation by cuprophane and other cellulose membranes, reactions to AN-69 together with ACE inhibitors, among others. In recent years, we have observed some patients’ reactions to synthetic membranes with PVP, which disappear once the dialysers are changed to cellulose triacetate.12,13 Currently there are asymmetrical high-flow cellulose triacetate dialysers, with a UFC of 87ml/mmHg/hr very suitable for OL-HDF.14 The new dialysers should solve problems derived from the interactions of the membrane with blood; as an example, avoiding the use of ETO for dialyser sterilisation. In addition, the material used for the dialyser casing and those required for its assembly may lead to adverse reactions or toxicity. Lastly, other toxic substances such as bisphenol A (BPA) should be avoided.15,16
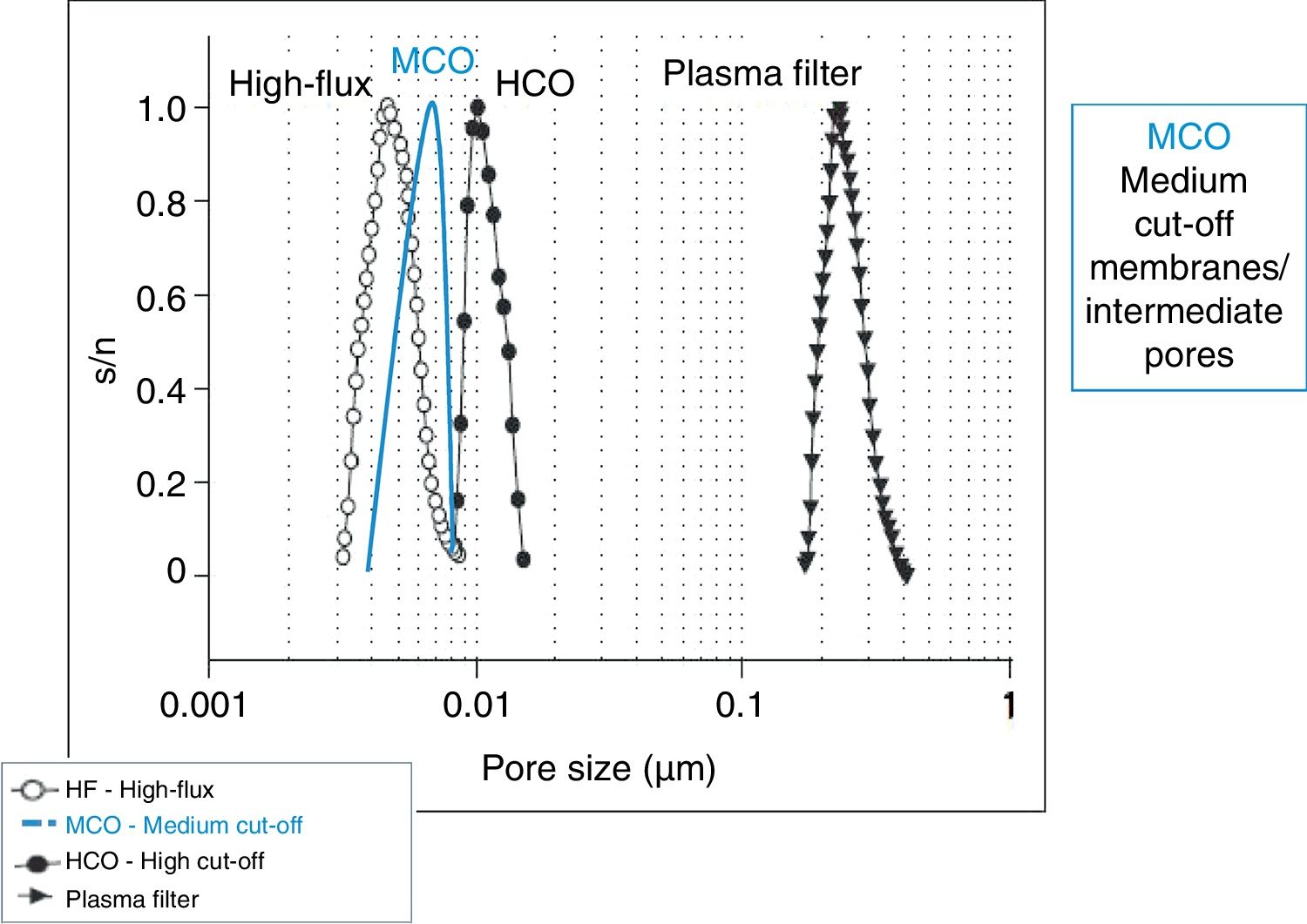
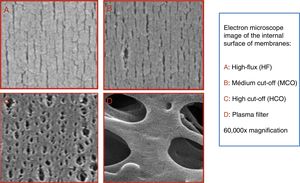
In recent years, dialysers have been specialised according to the dialysis technique. High cut-off (HCO) dialysers with large pores are specifically designed to remove large amount of molecules such as immunoglobulin light chains, in the treatment of myeloma-related kidney disease.17,18 Medium cut-off membranes (MCO) have recently been developed (Fig. 1), which are able to clear molecules such as HCO, but are able to retain albumin (Fig. 2).3 The clearance of middle molecule is greater with these dialysers than with high-flux (HF) dialysers.19
Some dialysers have been designed to take advantage of retrofiltration as a form of clearance via internal convective transport,20 known as internal haemodiafiltration (iHDF).21 One method to increase the iHDF is to raise the capillary density to ≥74%, attaining an iHDF of 3.5l/hr.22 Another method is to reduce the internal diameter of capillaries, which increases internal resistance, thus attaining greater retrofiltration. The internal diameter has been reduced of below 200μm to reach a diameter as small as 180μm, such as in the Theranova™ dialyser. It should be noted that the internal radius of the capillary appears to the power of four in the Hagen–Poiseuille equation, which is used for calculating blood resistance as it passes through capillaries. A long, narrow dialyser with a capillary diameter of 180–185μm will cause a very large pressure drop inside the capillary of around 200mmHg for a 500mlQb. This design would be ideal for a dialyser used for very high efficiency haemodialysis (HD-VHF). These dialysers are not recommended for OL-HDF. Instead, dialysers with an internal capillary diameter of greater than or equal to 200μm are recommended for OL-HDF. Therefore, the company Fresenius (FMC™) created a specific line of FXclass® dialysers—such as the Fx800® model—which is different from the Fx80®. In this edition of Nephrology, a study published by Dr Maduell et al.23 demonstrate a reduction rates of small and medium molecules that are similar in post-dilution OL-HDF with an automatic substitution mode, comparing dialysers FX60 vs. FX600 and FX80 vs. FX800. In OL-HDF; the clearance of medium molecules essentially depends on the ultrafiltered volume and the SC of each molecule. The two types of dialysers obtain a similar clearance. The study was conducted under optimal conditions: Qb of 450ml/min, 283minutes per session with an average of 32.5l UF per session; this means an average filtration fraction of 25.5%, which is not very high, with an average haematocrit of 29%. The dialysers recommended for post-dilution OL-HDF are not designed to achieve greater UF volume and higher molecule clearance; rather, they are designed to prevent haemoconcentration-related complications in less favourable conditions and with great filtration fractions of around 30%. We published a study24 comparing four dialysers in post-dilution OL-HDF: FX1000®, FX800®, Polyflux 210H® and Elisio 210H®. The first three were designed for OL-HDF and the latter for HF-HD. The rate of reduction in the concentration of the assessed molecules was similar across the four dialysers. This was also the case for the ultrafiltered volume. The difference observed was in relation with the number of alarms/problems of the dialysis machine occurring with the Elisio® dialyser. This was due to an increase in the pre-dialysis pressure, which was more than 100mmHg higher than in the other three dialysers. In our study the Qb values were lower, and three patients were dialyzed through a catheter as the vascular access. The selection of the best dialysers for OL-HDF has already been addressed.25,26 Therefore, the conclusions drawn from the Maduell et al. study cannot be extrapolated to dialysis performed in less favourable conditions.
The adsorption capacity of some membranes, such as polymethyl methacrylate (PMMA), has also improved, reducing the ability to activate the platelets and the thrombogenicity.27 Others have used this adsorption capacity to attach heparin and reduce thrombogenicity.28 The use with a citrate DF achieves promising results, avoiding the need for anticoagulation in HD.28
Other aspects to take into account in the dialyser are: volume of the blood compartment (optimal values are lower than <120ml for 2m2) and a residual blood volume of <1ml. The surface of the dialyser should depend on the Qb attained. Sterilisation: ETO has been dismissed as a sterilisation method for dialysers. Water vapour and gamma radiation are the most common methods currently used. When evaluating the data dialyser, we should ensure that the in vitro tests adhere to standard specifications, such as the ISO 8637: 2014.
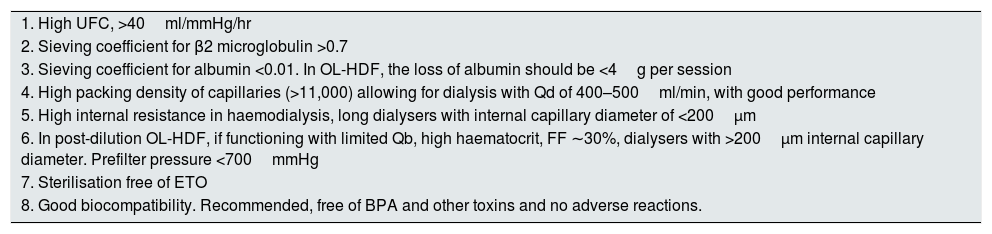
Table 1 outlines the characteristics that should guide the election of dialyser for a specific dialysis technique in a given patient. Some of these characteristics may be considered as quality criteria of a dialyser.
Recommended dialyser characteristics for conducting haemodialysis or haemodiafiltration (current optimal values).
| 1. High UFC, >40ml/mmHg/hr |
| 2. Sieving coefficient for β2 microglobulin >0.7 |
| 3. Sieving coefficient for albumin <0.01. In OL-HDF, the loss of albumin should be <4g per session |
| 4. High packing density of capillaries (>11,000) allowing for dialysis with Qd of 400–500ml/min, with good performance |
| 5. High internal resistance in haemodialysis, long dialysers with internal capillary diameter of <200μm |
| 6. In post-dilution OL-HDF, if functioning with limited Qb, high haematocrit, FF ∼30%, dialysers with >200μm internal capillary diameter. Prefilter pressure <700mmHg |
| 7. Sterilisation free of ETO |
| 8. Good biocompatibility. Recommended, free of BPA and other toxins and no adverse reactions. |
Technological advances have allowed dialysers to evolve significantly in recent years, making haemodialysis more effective, more efficient and safer. Just as we are individualising the prescription of DF, the current strategy is to match the choice of dialyser to the technique and the characteristics of the vascular access in each patient. Improvements have been made in the biocompatibility of membranes and dialysers, but there is still room for improvement so the risk of adverse effects are reduced further.
Pores are functionally changed due to several factors, but mainly due to contact with blood and the pressure applied to the membrane.
Key points- 1
Technological advancements of the dialyzers allow to match the choice of dialyser to the dialysis technique and the characteristics of the vascular access in each patient.
- 2
We should move from High Flux haemodialysis to iHDF. iHDF should not be considered a competitor for OL-HDF. Rather, it should be an alternative to take advantage of the benefits of convective transport in patients who are not susceptible to OL-HDF, it would have fewer technical requirements with less cost. The clinical benefits are still unknown.
- 3
In non-optimal situations, and with filtration fractions of 30%, OL-HDF requires specific dialysers.
- 4
Current dialysers should prevent adverse reactions, including hypersensitivity and toxic reactions. Biocompatibility should be improved.
- 5
In iHDF and OL-HDF, albumin losses should be small; the SCs should be below 0.01 and less than 4g per session.
Please cite this article as: Pérez-García R, Alcázar R. El dializador en el año 2017: mucho más que una membrana. Nefrologia. 2018;38:4–7.