Sclerostin is an anti-anabolic protein synthesized by osteocytes that may cause osteoporosis by inhibiting bone formation. The aim of our study was to investigate the correlation between sclerostin and bone mineral density (BMD) reduction in renal transplant recipients (RTRs) with more than 1 year after transplantation.
Material and methodsThis cross-sectional study was conducted on 80 patients (38 (47.5%) male/42 (52.5%) female) RTRs with a mean age of 44.68±10.39 years. Patients were compared with an age and sex-matched control group of 40 healthy individuals. BMD was measured by dual-energy X-ray absorptiometry. The levels of sclerostin were determined using enzyme-linked immunosorbent assay.
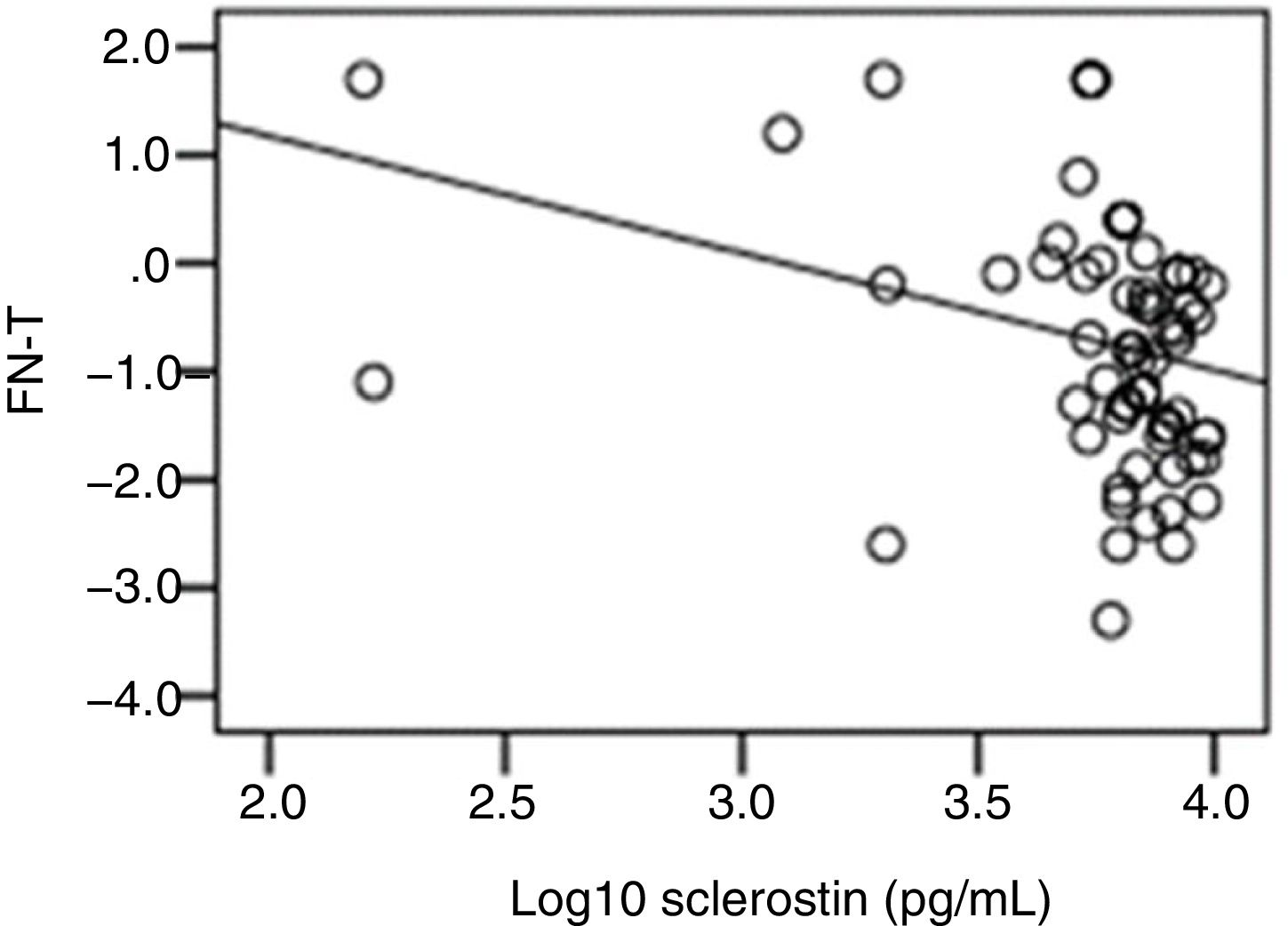
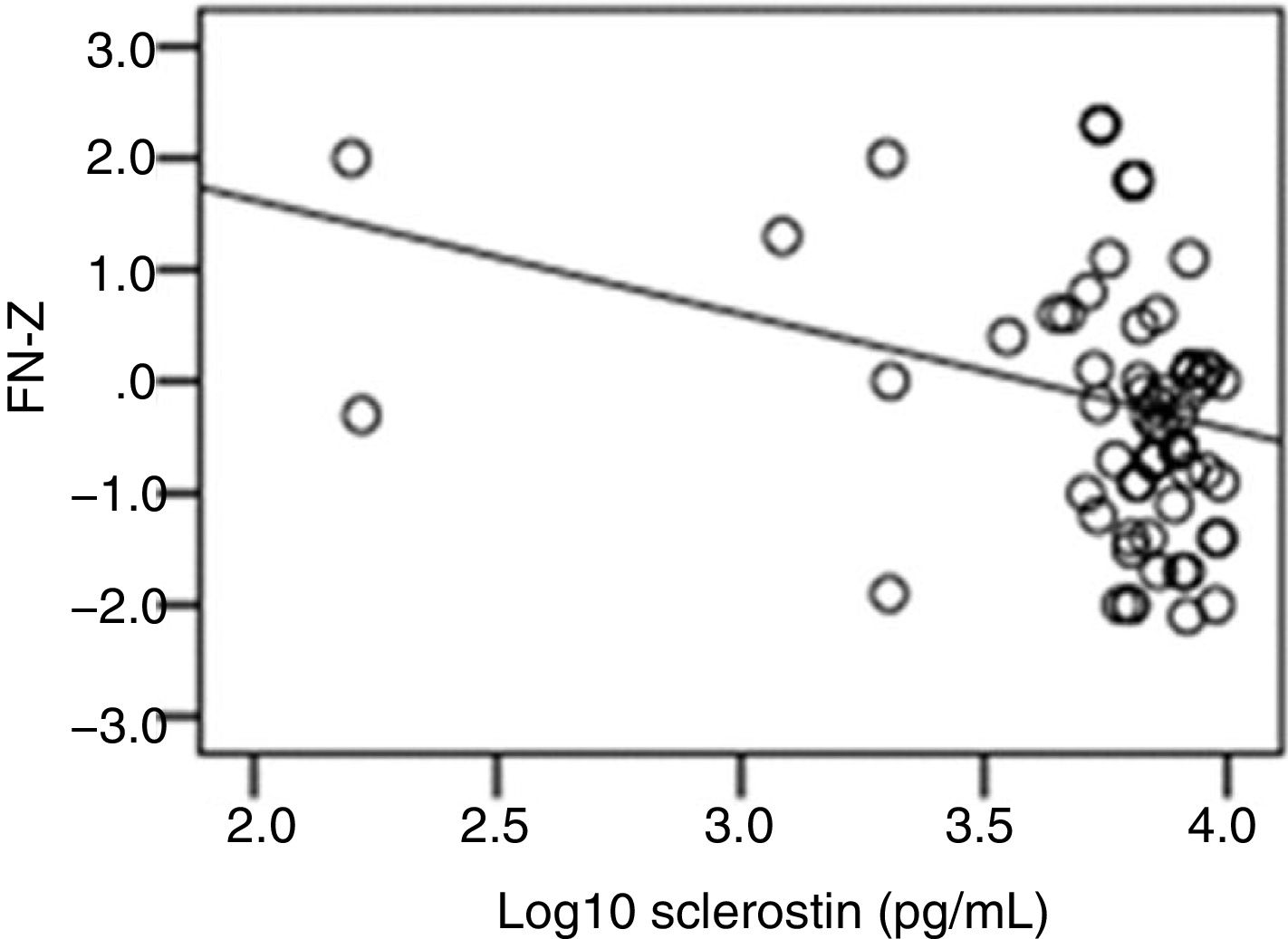
ResultsThe mean sclerostin was 3.77±0.3pg/mL in patients and 3.81±0.21pg/mL in healthy individuals. The mean T score of femoral trochanter (FT) (FT-T), femoral neck (FN) (FN-T), lumbar vertebrae (L1-4) (L1-4-T) were −0.81±0.86, −1.08±1.09 and −0.8±1.2, respectively. The mean Z score of FT (FT-Z), FN (FN-Z), L1-4 (L1-4-Z) were −0.6±0.73, −0.32±0.9 and −0.54±1.13, respectively. FT-Z and L1-4-Z were lower in patients than healthy subjects (p=0.009, p=0.021 respectively). Serum creatinine (p<0.001), intact parathyroid hormone (p<0.001) were higher and phosphate (p<0.001), was lower in patients than healthy subjects. Patients with a log10 sclerostin of >3.84pg/mL had higher FT-T (p=0.040), FT-Z, FN-T (p=0.018), FN-Z (p=0.006) than those with a log10 sclerostin of ≤3.84pg/mL. There was a significant correlation between log10 sclerostin and FN-T (r=−0.296, p=0.009) and FN-Z (r=−0.269, p=0.019). In linear regression analysis, high sclerostin was found to be correlated with male gender, lower FN-T and lower FN-Z independently of other risk factors.
ConclusionThe levels of sclerostin can predict reduction of proximal femur BMD and development of mineral and bone disorder in RTRs. There was no difference in sclerostin levels between RTRs and healthy individuals.
La esclerostina es una proteína con efecto antianabólico sintetizada por los osteocitos que puede causar osteoporosis al inhibir la formación de hueso. El objetivo de nuestro estudio fue investigar la correlación entre la esclerostina y la reducción de la densidad mineral ósea (DMO) en receptores de trasplante renal (RTR) más de un año después del trasplante.
Materiales y métodosEste estudio transversal se realizó en 80 pacientes (38 [47,5%] varones/42 [52,5%] mujeres) RTR con una edad media de 44,68±10,39 años. Se comparó a los pacientes con un grupo de comparación emparejado por edad y sexo de 40 individuos sanos. La DMO se midió mediante absorciometría de rayos X de doble energía. Los niveles de esclerostina se determinaron utilizando un enzimoinmunoanálisis de adsorción.
ResultadosEl nivel medio de esclerostina fue de 3,77±0,3pg/ml en pacientes y 3,81±0,21pg/ml en individuos sanos. La puntuación T media del trocánter femoral (TF) (T-TF), del cuello femoral (CF) (T-CF), las vértebras lumbares (L1-4) (T-L1-4) fue de −0,81±0,86, −1,08±1,09 y −0,8±1,2, respectivamente. La puntuación Z media del TF (Z-TF), CF (Z-CF), L1-4 (Z-L1-4) fue de –0,6±0,73, −0,32±0,9 y −0,54±1,13, respectivamente. Las puntuaciones Z-TF y Z-L1-4 fueron inferiores en los pacientes que en los sujetos sanos (p=0,009 y p=0,021, respectivamente). Los niveles de creatinina sérica (p<0,001) y hormona paratiroidea intacta (p<0,001) fueron superiores en los pacientes que en los sujetos sanos, y los niveles de fosfato (p<0,001) fueron inferiores. Los pacientes con un log10 esclerostina >3,84pg/ml tuvieron puntuaciones T-TF (p=0,040), Z-TF, T-CF (p=0,018), Z-CF (p=0,006) superiores a las de los pacientes con un log10 esclerostina ≤3,84pg/ml. Se observó una correlación significativa entre log10 esclerostina y T-CF (r=−0,296, p=0,009) y Z-CF (r=−0,269, p=0,019). En el análisis de regresión lineal, se observó que los niveles elevados de esclerostina estaban correlacionados con el sexo masculino, una puntuación T-CF inferior y una puntuación Z-CF inferior independientemente de otros factores de riesgo.
ConclusiónLos niveles de esclerostina pueden predecir la reducción de la DMO del fémur proximal y el desarrollo de un trastorno mineral y óseo en RTR. No se observaron diferencias en los niveles de esclerostina entre los RTR y los individuos sanos.
In chronic kidney disease (CKD) patients the reduction of renal function may be accompanied by loss of bone mineral density.1 The development of CKD-MBD is associated with increased morbidity-mortality and risk of bone fracture. Renal transplant recipients (RTRs) are at high risk of decreased bone mineral density (BMD) and CKD-MBD development due to the intense use of immunosuppressive drugs, presence of a previous bone lesion, and long term history of CKD.2 In the early posttransplant period, the risk of bone fracture is 7 times higher as compared to healthy individuals and 30% higher than patients with end-stage renal disease (ESRD).3
Wingless-type mouse mammary tumor virus integration site (Wnt) plays a significant role in the regulation of bone metabolism.4 The (canonical) Wnt-β-catenin signal pathway is the predominant component of Wnt signal which has a role in the regulation of bone cell activity.5 Sclerostin is an anti-anabolic glycoprotein which is synthesized from osteocytes, and inhibits the Wnt-β-catenin signaling pathway required for osteoblast development and activity.6 The inhibition of Wnt-β-catenin signaling pathway receptors by sclerostin is thought to result in a decrease in the local skeletal activity.7
Brandenburg et al. suggested that sclerostin can be used as a new CKD-MBD-related marker in CKD patients.8 Thambiah et al. reported that serum sclerostin levels were associated with CKD-MBD development in CKD patients.9 Ishimura et al. reported that there was an inverse correlation between sclerostin and bone turnover markers in ESRD patients on dialysis and that sclerostin showed an anti-anabolic effect by inhibiting osteoblastic activity.10 Cejka et al. reported that sclerostin is inversely correlated with osteoblast number and histologicparameters of bone turn-over in ESRD patients on dialysis11; in another study they show a correlation between sclerostin and reduction of BMD in dialysis patients.12
Sabbagh et al. showed in mice with polycystic kidney disease an early increase in bone synthesis of sclerostin.13 Pelletier et al. reported that the serum sclerostin levels of CKD patients were 3–4 times higher compared to healthy individuals. The same study reported that as the renal functions of CKD patients decreased, there was an increase in their serum sclerostin levels.14 Kanbay et al. reported that in predialysis CKD patients as renal functions of deteriorated, the serum sclerostin levels increased by 2–4 folds compared to that of healthy individuals due to decreased renal clearance or increased skeletal system production.15 In the study by Cejka et al. on predialysis CKD patients, it was reported that excretion of sclerostin was 10 times higher in stage 5 CKD patients than in stage 1 CKD patients. It was suggested that the reason for high serum sclerostin levels in CKD patients might be due to increased bone production of sclerostin.16
It is important to prevent long-term complications such as decreased BMD and development of MBD during the post-transplant period.17 Although there are many studies investigating the correlation between sclerostin and MBD in predialysis and dialysis CKD patients, studies on RTRs is limited, and their results are contradictory. The aim of our study is to investigate the correlation between sclerostin sclerostin and BMD as determined by dual-energy X-ray absorptiometry (DXA) in RTRs with ≥1 year post-transplantation maintained on low-dose steroid.
Materials and methodsPatient selectionThis is cross-sectional study was conducted on 80 cadaveric or living-donor transplantation in the Renal Transplantation Outpatient Clinic of Yuksek Ihtisas Training and Research Hospital between February 2018 and January 2019. The patients were compared with 40 age- and sex-matched healthy individuals with no history of any known comorbid disease or need of medication.
The study included patients >18 years who agreed to participate in the study, with functional graft for more than ≥1 year post-transplantation without rejection or need of dialysis. Patients exclude were those who did not accept to participate, had active malignancy and/or systemic disease that could affect the interpretation of the data, such as severe infection, immobilization, primary bone disease, history of severe osteoporosis (T score of ≤−2.5) and one or more fractures of the proximal femur [femoral trochanter (FT), femoral neck (FN)] or the lumbar vertebrae (1–4), history of severe or unremitting secondary hyperparathyroidism or parathyroidectomy since serum the levels of parathyroid hormone (PTH) may affect the production of sclerostin, and those using drug affecting bone mineral metabolism (oral phosphate (P) binders, calcimimetics, active vitamin D or vitamin D analog) were excluded from the study. All patients were given triple therapy containing mycophenolate mofetil (MMF)/mycophenolate sodium (MNa) and steroids with calcineurin inhibitor (CNI) (cyclosporine or tacrolimus)/mammalian target of rapamycin (mTOR) inhibitor (sirolimus or everolimus) as immunosuppressive therapy. Induction therapy was carried out with basiliximab or anti-thymocyte globulin.
The aim of the study was explained to all participants, and written informed consent was obtained from those who agreed to participate. The approval for the study was obtained from the Ethics Committee of the Yuksek Ihtisas Training and Research Hospital.
Data collection and laboratory measurementsVenous blood samples taken following 10–12h of nightfasting were centrifuged at 4°C for 10min, the supernatants were stored at −80°C. Serum creatinine, calcium (Ca), and phosphate (P) levels were measured with the spectrophotometric method using Beckman Coulter commercial kits on Beckman Coulter AU5800 (Beckman Coulter Instrumentation, CA, USA) autoanalyzer. Intact parathyroid hormone (iPTH) was measured using the chemiluminescence method on Beckman Coulter DxI800 (Beckman Coulter Inc, San Diego, CA, USA) analyzer. 25 hydroxy(OH)vitamin(Vit) D3 (25(OH)VitD3) level was measured with chemiluminescence assay using Liason (DiaSorin, MN, USA) analyzer. Serum sclerostin (Elabscience, CA, USA) levels were measured with enzyme-linked immunosorbent assay (ELISA) kits. For all parameters, the inter- and intra-assay coefficients of variations were <10%; detection range and analytical sensitivity values were 62.50–4000pg/mL and 37.50pg/mL for sclerostin, respectively. Estimated glomerular filtration rate (eGFR) value was determined by the Modification of Diet in Renal Disease (MDRD)18 method.
Dual-energy X-ray absorptiometry (DXA)Total femur (FT) and femoral neck (FN) BMD measurement for determining fracture risk and lumbar (L) BMD measurement are the most useful parameters for treatment follow-up. In our study, T score of FT (FT-T), FN (FNT), L1-4 (L1-4-T) and Z score of FT (FT-Z), FN (FN-Z), L1-4 (L1-4-Z) BMD measurements were made by dual-energy X-ray absorptiometry (DXA) device (HOLOGIC Instrumentation, Waltham, USA) instrument. The total L BMD was calculated by averaging the BMD of vertebrae L1 to L4. BMD results were determined as g/cm2 and the T and Z scores were analyzed to determine fracture risk. The T-score was defined as the standard deviation of the comparison of the individual's bone mass value with the reference peak bone mass of healthy young adults, and the Z-score was defined as the standard deviation of the comparison of the individual's bone mass value with the reference value of healthy individuals in his/her own age, sex and race groups.19 According to the diagnostic criteria for osteoporosis defined by the World Health Organization (WHO), a T score of >−1 was considered as normal, between −1 and −2.5 as osteopenia, and a T score of ≤−2.5 was considered as osteoporosis development.
Statistical analysisThe statistical analysis was made using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY). The data are expressed as n (%), mean±standard deviation ormedian (minimum–maximum), as appropriate. Pearson chi-square analysis was performed for categorical variables. The normality assumptions were tested by the Shapiro–Wilk test. The Mann–Whitney U test and Student's t-test were used for the analysis of non-normally and normally distributed numerical data, respectively. The Pearson correlation test was applied to analyze correlation between the continuous variables. Univariate and multivariate linear regression analysis was performed to determine the independent factors associated with log10 sclerostin. Univariate analysis was used to determine all variables that are considered to be associated with the variable log10 sclerostin including age, gender, time in post-transplant period, immunosuppressive drugs used, eGFR and BMD T and Z scores. All factors with p<0.02 on univariate analysis (age, gender, Tacrolimus-MMF/MNa-steroid, Sirolimus-MMF/MNa-steroid, FN-T, FT-Z, FN-Z) were analyzed in the multivariate model. The multivariate linear regression analysis requires that the errors between observed and predicted values (i.e., the residuals of the regression) should be normally distributed. For this reason, log transformation was applied to sclerostin variable. Since FN-T and FN-Z measurements are strongly correlated, separate regression models (Model 1 for FN-T, Model 2 for FN-Z) were created for each variables. p values of <0.05 were considered statistically significant.
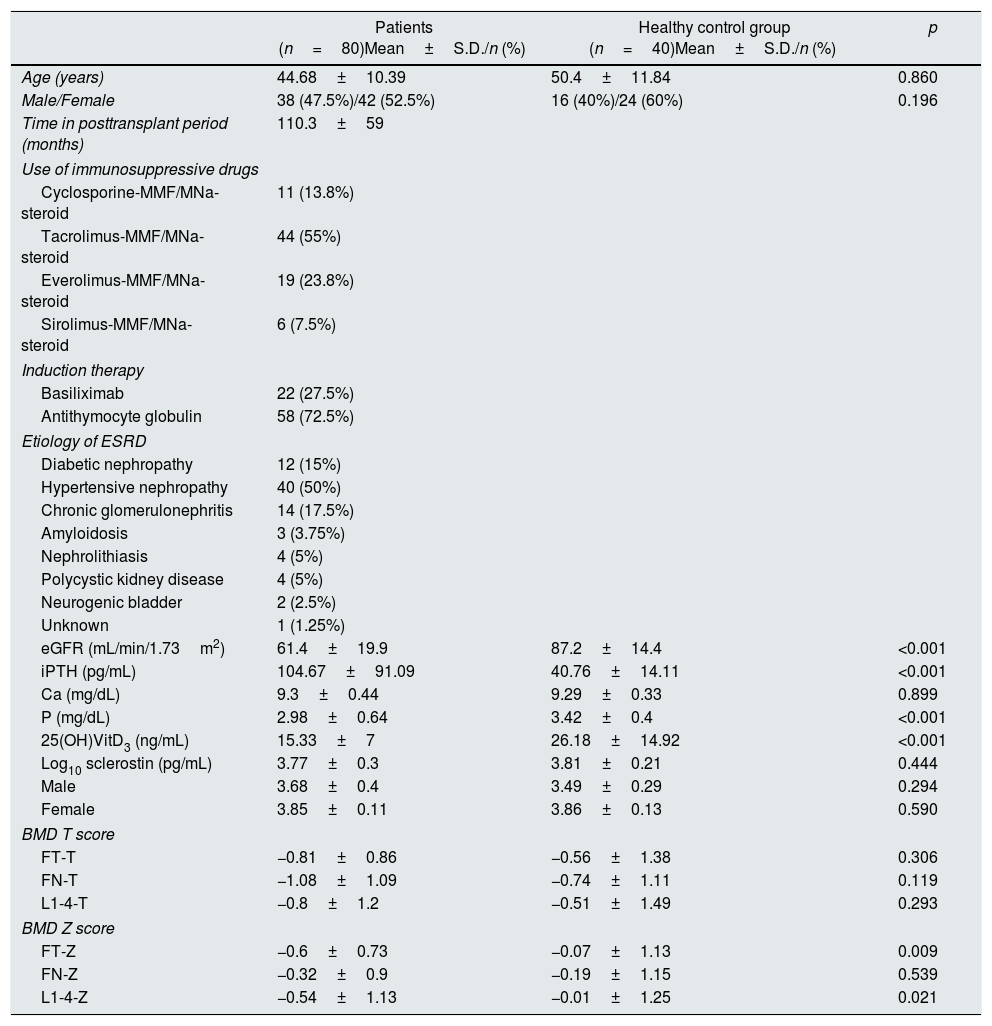
ResultsPatients’ characteristicsThe study was conducted on a total of 80 renal transplant recipients, 38(47.5%) male and 42 (52.5%) female with a mean age of 44.68±10.39 years (Table 1). The mean post-transplant period was 110.3±59 months. Eleven patients (13.8%) were on cyclosporine–MMF/MNa–steroid, 44 (55%) on tacrolimus–MMF/MNa–steroid, 19 (23.8%) on everolimus–MMF/MNa–steroid and 6 (7.5%) on sirolimus–MMF/MNa–steroid. The etiology of ESRD was diabetic nephropathy (n=12), hypertensive nephropathy (n=40), chronic glomerulonephritis (n=14), amyloidosis (n=3), nephrolithiasis (n=4), polycystic kidney disease (n=4), neurogenic bladder (n=2) and unknown (n=1). The mean creatinine was 1.35±1.17mg/dL and the mean eGFR was 61.4±19.9mL/min/1.73m2. The mean iPTH, Ca, P, 25(OH)VitD3 were 104.7±91.1pg/mL, 9.3±0.4mg/dL, 2.98±0.64mg/dL, and 15.33±7ng/mL, respectively. The mean log10 sclerostin was 3.77±0.3pg/mL. The mean FT-T, FT-Z, FN-T, FN-Z, L1-4-T, L1-4-Z were −0.81±0.86, −0.6±0.73, −1.08±1.09, −0.32±0.9, −0.8±1.2 and −0.54±1.13, respectively. Patients were compared with 40 healthy subjects. The patients had significantly higher iPTH (p<0.001) and significantly lower eGFR, P, 25(OH)VitD3 (p<0.001), FT-Z (p=0.009), L1-4-Z (p=0.021) as compared to the healthy individuals. There was no difference between both groups with regard to log10 sclerostin (p>0.05) (Table 1).
Clinical, demographic characteristics, laboratory and BMD T–Z values of the patients and the healthy control group.
| Patients (n=80)Mean±S.D./n (%) | Healthy control group (n=40)Mean±S.D./n (%) | p | |
|---|---|---|---|
| Age (years) | 44.68±10.39 | 50.4±11.84 | 0.860 |
| Male/Female | 38 (47.5%)/42 (52.5%) | 16 (40%)/24 (60%) | 0.196 |
| Time in posttransplant period (months) | 110.3±59 | ||
| Use of immunosuppressive drugs | |||
| Cyclosporine-MMF/MNa-steroid | 11 (13.8%) | ||
| Tacrolimus-MMF/MNa-steroid | 44 (55%) | ||
| Everolimus-MMF/MNa-steroid | 19 (23.8%) | ||
| Sirolimus-MMF/MNa-steroid | 6 (7.5%) | ||
| Induction therapy | |||
| Basiliximab | 22 (27.5%) | ||
| Antithymocyte globulin | 58 (72.5%) | ||
| Etiology of ESRD | |||
| Diabetic nephropathy | 12 (15%) | ||
| Hypertensive nephropathy | 40 (50%) | ||
| Chronic glomerulonephritis | 14 (17.5%) | ||
| Amyloidosis | 3 (3.75%) | ||
| Nephrolithiasis | 4 (5%) | ||
| Polycystic kidney disease | 4 (5%) | ||
| Neurogenic bladder | 2 (2.5%) | ||
| Unknown | 1 (1.25%) | ||
| eGFR (mL/min/1.73m2) | 61.4±19.9 | 87.2±14.4 | <0.001 |
| iPTH (pg/mL) | 104.67±91.09 | 40.76±14.11 | <0.001 |
| Ca (mg/dL) | 9.3±0.44 | 9.29±0.33 | 0.899 |
| P (mg/dL) | 2.98±0.64 | 3.42±0.4 | <0.001 |
| 25(OH)VitD3 (ng/mL) | 15.33±7 | 26.18±14.92 | <0.001 |
| Log10 sclerostin (pg/mL) | 3.77±0.3 | 3.81±0.21 | 0.444 |
| Male | 3.68±0.4 | 3.49±0.29 | 0.294 |
| Female | 3.85±0.11 | 3.86±0.13 | 0.590 |
| BMD T score | |||
| FT-T | −0.81±0.86 | −0.56±1.38 | 0.306 |
| FN-T | −1.08±1.09 | −0.74±1.11 | 0.119 |
| L1-4-T | −0.8±1.2 | −0.51±1.49 | 0.293 |
| BMD Z score | |||
| FT-Z | −0.6±0.73 | −0.07±1.13 | 0.009 |
| FN-Z | −0.32±0.9 | −0.19±1.15 | 0.539 |
| L1-4-Z | −0.54±1.13 | −0.01±1.25 | 0.021 |
Data are presented as n (%), mean±SD and median (min–max). Mann–Whitney U test, Student's t test, Pearson chi-square test, Fisher's Exact test. Abbreviations: MMF, mycophenolate mofetil; MNa, mycophenolate sodium; ESRD, end-stage renal disease; iPTH, intact parathyroid hormone; Ca, calcium; P, phosphate; 25(OH)VitD3, 25 hydroxy(OH)vitamin(Vit) D3; BMD; bone mineral density; FT, femoral trochanter; FN, femoral neck; L, lumbar vertebrae; SD, standard deviation.
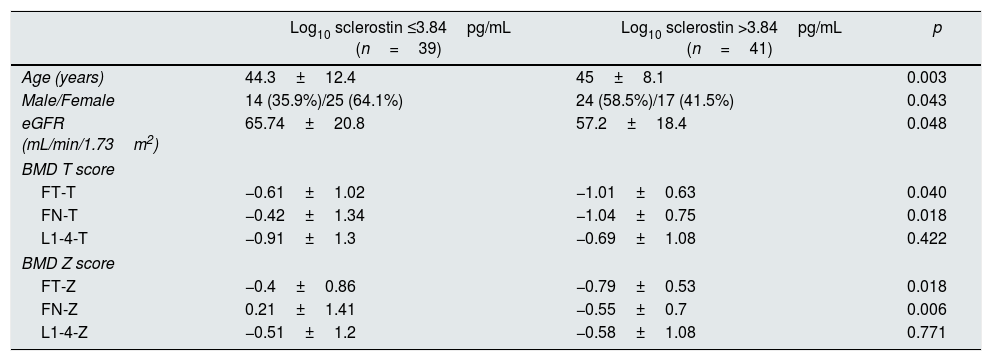
The median log10 sclerostin level was determined as 3.84pg/mL. Patients with log10 sclerostin level of >3.84pg/mL had more age (p=0.003), there were more males (p=0.004), the FT-T (p=0.040), FN-T (p=0.018), FN-T (p=0.018) and FN-Z (p=0.006) were significantly higher and eGFR (p=0.048) was significantly lower than in those with a log10 sclerostin level of ≤3.84pg/mL. Since the log10 sclerostin variable was grouped according to the median value, there was no change in the data of the patients in both groups when the logarithmic transformation was not applied to the sclerostin variable (Table 2).
Comparison of patient characteristics according to median log10 sclerostin.
| Log10 sclerostin ≤3.84pg/mL (n=39) | Log10 sclerostin >3.84pg/mL (n=41) | p | |
|---|---|---|---|
| Age (years) | 44.3±12.4 | 45±8.1 | 0.003 |
| Male/Female | 14 (35.9%)/25 (64.1%) | 24 (58.5%)/17 (41.5%) | 0.043 |
| eGFR (mL/min/1.73m2) | 65.74±20.8 | 57.2±18.4 | 0.048 |
| BMD T score | |||
| FT-T | −0.61±1.02 | −1.01±0.63 | 0.040 |
| FN-T | −0.42±1.34 | −1.04±0.75 | 0.018 |
| L1-4-T | −0.91±1.3 | −0.69±1.08 | 0.422 |
| BMD Z score | |||
| FT-Z | −0.4±0.86 | −0.79±0.53 | 0.018 |
| FN-Z | 0.21±1.41 | −0.55±0.7 | 0.006 |
| L1-4-Z | −0.51±1.2 | −0.58±1.08 | 0.771 |
Data are presented as n (%), mean±SD and median (min–max). Mann–Whitney U test, Student's t test, Pearson chi-square test, Fisher's Exact test.
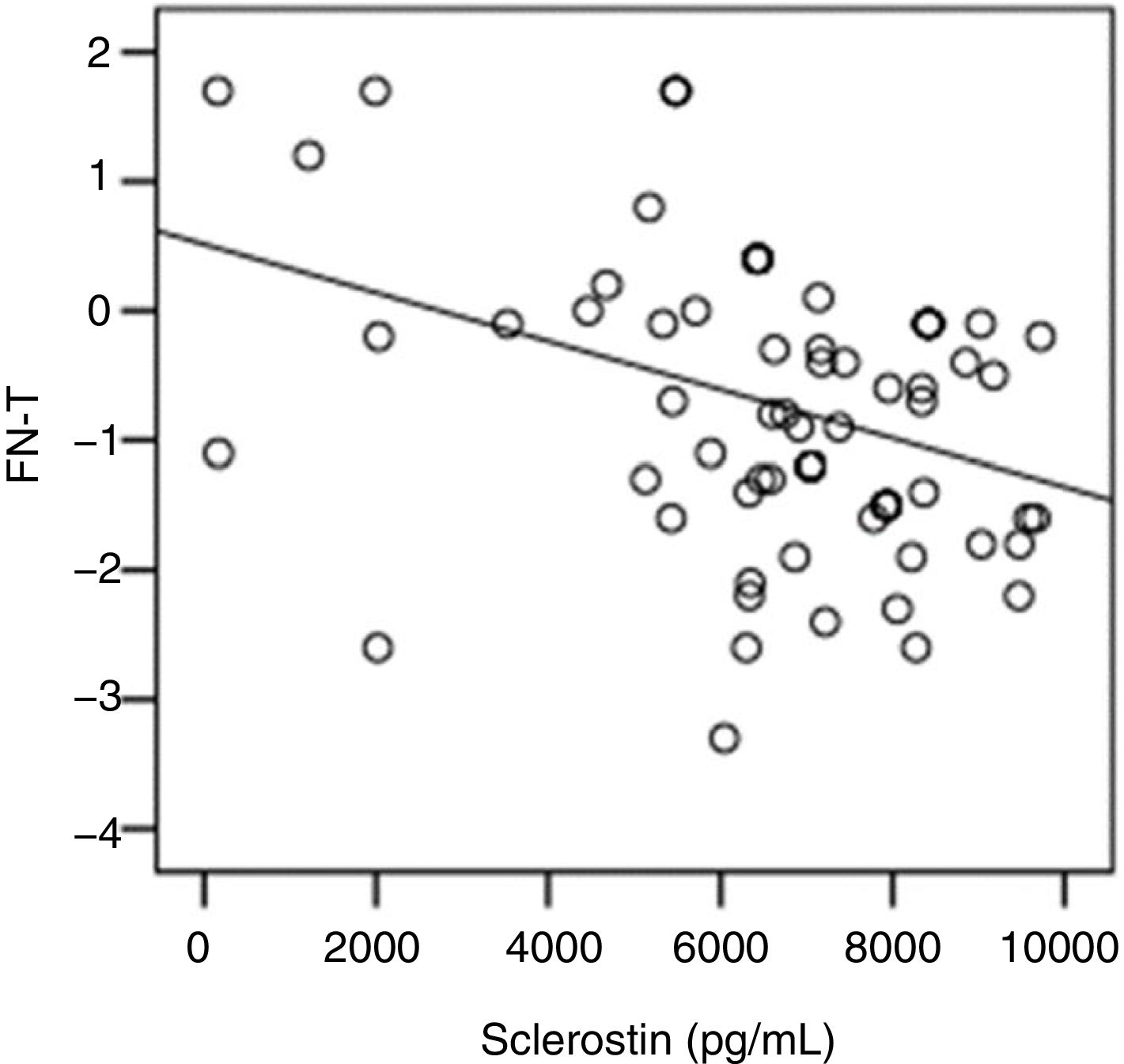
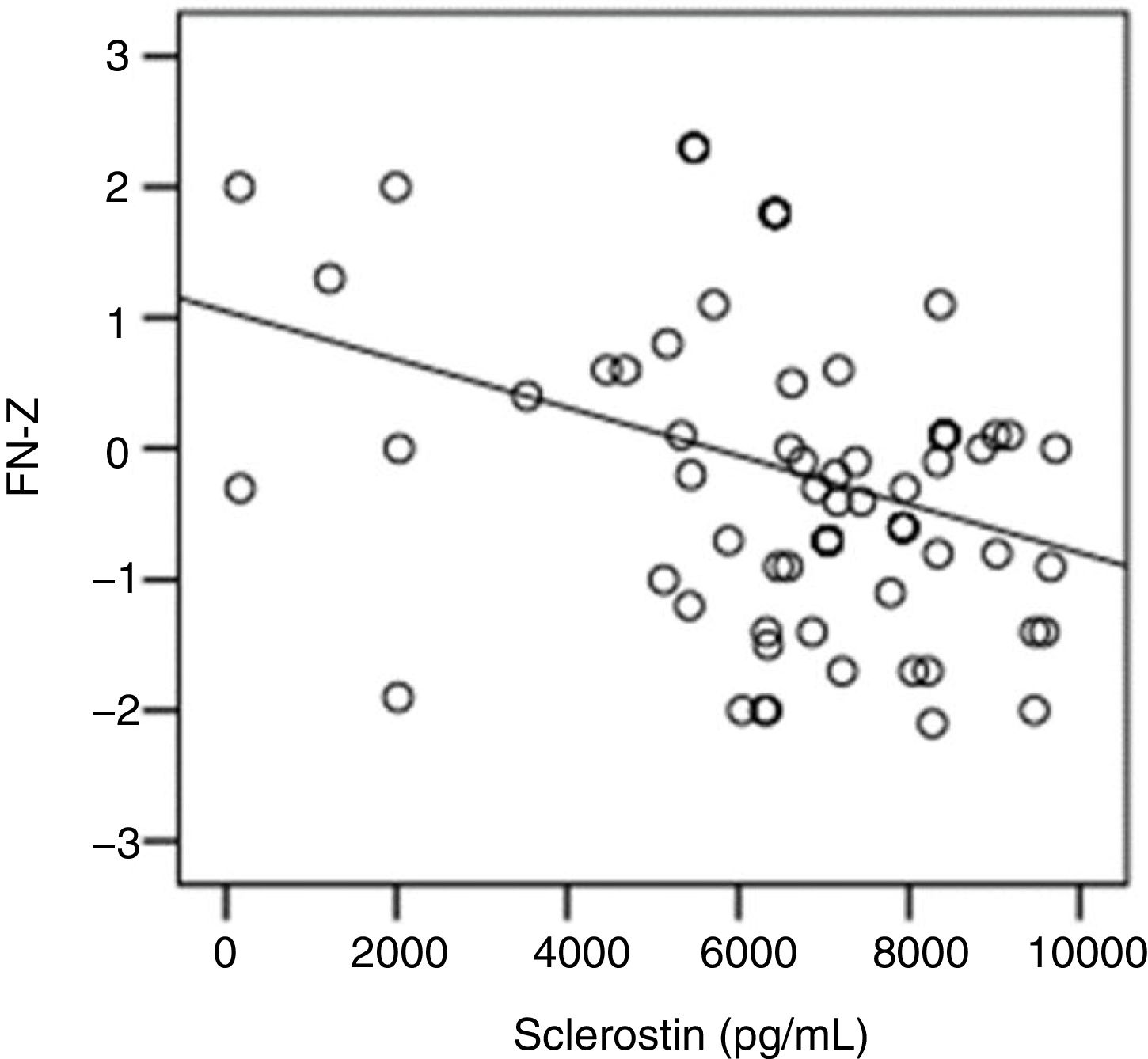
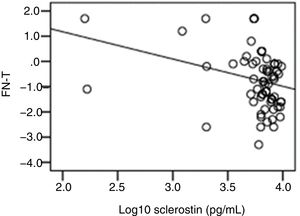
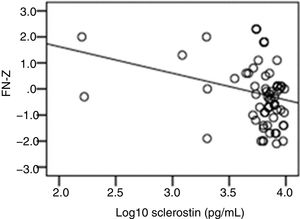
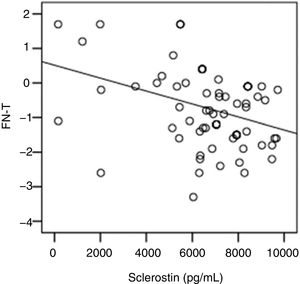
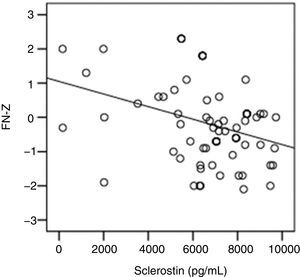
The correlation analysis showed a significant inverse correlation between log10 sclerostin and FN-T BMD (r=−0.296, p=0.009) (Fig. 1) and FN-Z BMD (r=−0.269, p=0.019) (Fig. 2). Also the correlation analysis shows a significant inverse correlation between sclerostin and FN-T BMD (r=−0.292, p=0.010) (Fig. 3) and FN-Z BMD (r=−0.245, p=0.033) (Fig. 4). There was no significant correlation between log10 sclerostin and eGFR (r=−0.112, p=0.323), FT-T (r=−0.118, p=0.301), FTZ (r=−0.179, p=0.115), L1-4-T (r=−0.049, p=0.668), L1-4-Z (r=−0.102, p=0.372) BMD reduction.
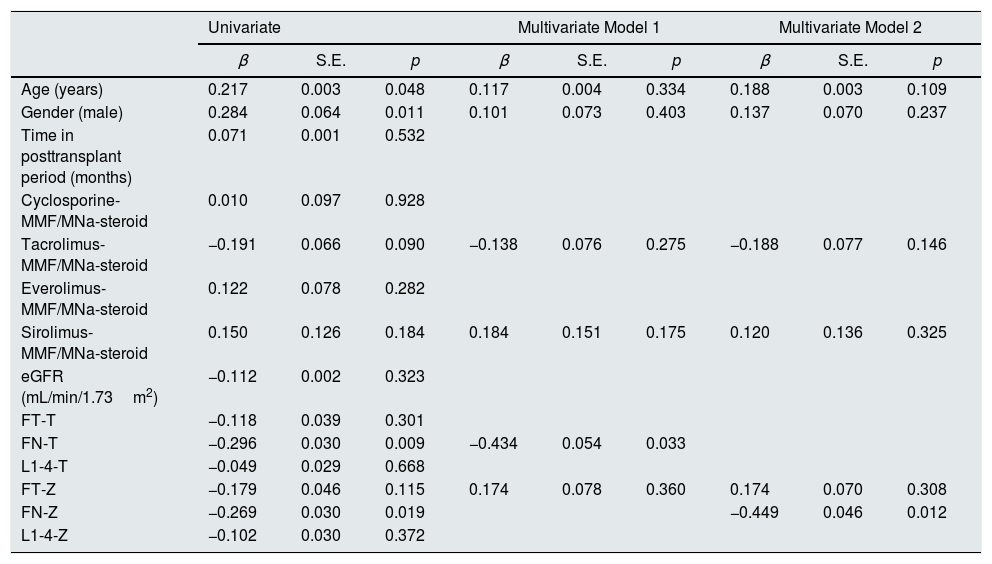
In Table 3, linear regression analysis was used to determine the factors affecting log10 sclerostin variable. The dependent variable log10 sclerostin was taken as a continuous variable. Variables such as age and gender were significant. A multivariate linear regression analysis was performed to determine independent risk factors associated with serum sclerostin level and it was found that low FN-T (p=0.033) (Model 1) and low FN-Z (p=0.012) (Model 2) were independently associated with high serum sclerostin levels independently of other risk factors (Table 3).
Correlation of log10 sclerostin with BMD T–Z score values in linear regression analysis.
| Univariate | Multivariate Model 1 | Multivariate Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | S.E. | p | β | S.E. | p | β | S.E. | p | |
| Age (years) | 0.217 | 0.003 | 0.048 | 0.117 | 0.004 | 0.334 | 0.188 | 0.003 | 0.109 |
| Gender (male) | 0.284 | 0.064 | 0.011 | 0.101 | 0.073 | 0.403 | 0.137 | 0.070 | 0.237 |
| Time in posttransplant period (months) | 0.071 | 0.001 | 0.532 | ||||||
| Cyclosporine-MMF/MNa-steroid | 0.010 | 0.097 | 0.928 | ||||||
| Tacrolimus-MMF/MNa-steroid | −0.191 | 0.066 | 0.090 | −0.138 | 0.076 | 0.275 | −0.188 | 0.077 | 0.146 |
| Everolimus-MMF/MNa-steroid | 0.122 | 0.078 | 0.282 | ||||||
| Sirolimus-MMF/MNa-steroid | 0.150 | 0.126 | 0.184 | 0.184 | 0.151 | 0.175 | 0.120 | 0.136 | 0.325 |
| eGFR (mL/min/1.73m2) | −0.112 | 0.002 | 0.323 | ||||||
| FT-T | −0.118 | 0.039 | 0.301 | ||||||
| FN-T | −0.296 | 0.030 | 0.009 | −0.434 | 0.054 | 0.033 | |||
| L1-4-T | −0.049 | 0.029 | 0.668 | ||||||
| FT-Z | −0.179 | 0.046 | 0.115 | 0.174 | 0.078 | 0.360 | 0.174 | 0.070 | 0.308 |
| FN-Z | −0.269 | 0.030 | 0.019 | −0.449 | 0.046 | 0.012 | |||
| L1-4-Z | −0.102 | 0.030 | 0.372 | ||||||
Model 1, all parameters with p<0.2 in univariate analysis, except FN-Z (R=0.451, R2=0.203, p=0.013). Model 2, all parameters with p<0.2 in univariate analysis, except FN-T (R=0.472, R2=0.223, p=0.007).
The present study shows a significant correlation between sclerostin and BMD reduction in RTRs. To minimize the effects of steroid on the BMD reduction, our study included patients with ≥1 year post transplantation and all patients were on 5mg/day of steroids.
It was found that serum sclerostin levels of RTRs were not different from that of healthy individuals. The study by Tomei et al. showed that the serum sclerostin levels it were similar in RTRs and CKD patients with similar eGFR.20 In the study by Tartaglione et al. it was reported that serum sclerostin levels in RTR were not different from that of healthy subjects.21
In our study, patients with high sclerostin levels were older and more frequent in males and this association was also confirmed by multivariate analysis. Mödder et al. reported that sclerostin levels increase with age and in males due to increased total body skeletal mass.22 The study by Tartaglione et al. on 80 RTRs, showed that males had higher serum sclerostin levels than females.21 They also suggested that this was due to the high body mass index in males or high serum estrogen levels in females that inhibit sclerostin production.
In our study patients with high sclerostin levels had lower eGFR values, however this was not confirmed by the correlation analysis and multivariate analysis. This result validate the view that serum sclerostin levels are not stronglyintensely modified by eGFR in RTRs. Bonani et al. reported that serum sclerostin levels decreased in the early period after transplantation to become normal by the end of the first year. They reported no association between serum sclerostin levels and eGFR and suggested that the cause of normalization of sclerostin levels was due to an improvement in serum Ca, P and vitamin D levels.23 In the study by Tartaglione et al. RTRs with a post-transplant period of more than 1 year and with a eGFR of >15mL/min/1.73m2, there was no correlation between sclerostin and eGFR, and this might be due to increased tubular excretion of sclerostin as eGFR decreases.21
The determination of BMD in RTRs by DXA is a useful test to evaluate risk of fracture risk.24 Although in ESRD patients there has been reported a relationship between sclerostin and CK-MBD, two studies in RTRs report the opposite. Tomei et al. showed that there was no relationship between sclerostin and bone turnover markers in a study including 19 postmenopausal RTRs. The limitations of this study were the low number of patients, low/moderate renal dysfunction in patients, and the absence of marked bone disease.20 Similarly, Bonani et al. reported that there was no association between sclerostin and reduction of BMD in RTRs, however although this association was seen in CKD patients. He argued that this was due to different demographic and biological features of patients such as younger age or preserved bone mass.23
In a preclinical study in mice, Ominsky et al. showed a relationship between sclerostin and FN BMD reduction and an increase in FN BMD after administration monoclonal antibody that inhibits sclerostin; the latter was due to an increase in osteoblast activity.25 Maluche et al. suggested that there was a correlation between sclerostin and FN BMD reduction in ESRD patients on dialysis and that sclerostin could be used as a new non-invasive marker for bone mass reduction.26 Since proximal femur and L1-4 measurements with DXA are known to be the most sensitive points showing BMD reduction, these regions were examined in our study, and it was found that there was a correlation between high serum sclerostin levels and FN BMD reduction independently of other risk factors. Our results were similar to the findings of a previous study21 and supports the opinion that sclerostin can be used as a marker of FN BMD reduction in RTRs. The decreased proximal femur BMD may be the highest risk of fracture as reported in RTRs.27
Serum PTH levels are thought to increase the production of sclerostin. In our study, RTRs had higher serum iPTH than healthy individuals and it was found that patients with elevated sclerostin levels had significantly increased iPTH levels. Makowka et al. reported that serum PTH levels in the post-transplant 9th month were still 3 times higher than normal.28 Torres et al. reported that resistant parathyroid hyperplasia and high serum PTH levels continued despite t good graft functions even after successful renal transplantation.29 Evenepoel et al. reported that the reason for still high post-transplant PTH levels may be related to the prolonged duration of dialysis before transplantation and the severity of secondary hyperparathyroidism.30 Kanaan et al., using computed tomography and bone turnover marker's reported that high PTH levels in the post-transplant period caused cortical and trabecular bone reduction in the peripheral skeletal system.31 High serum iPTH levels despite a post-transplant period of ≥1 year may be triggering sclerostin production from osteocytes and proximal femur BMD reduction.
Our study aimed to investigate the correlation between sclerostin and BMD reduction in RTRs, and although the results obtained were satisfactory for us, the presence of some limitations affecting the results are notable. First, the study was cross-sectional and the long-term effects of sclerostin on BMD reduction were not investigated. Second, the study was conducted on RTRs at ≥1 year post-transplantation. The correlation of sclerostin with BMD was not investigated in patients pretransplant and early post-transplant periods and using high-dose of steroids. Third, patients were compared with healthy individuals; CKD patients with similar age and eGFR values were not included in the study. Fourth, in addition to sclerostin, the correlation of other bone turnover markers, fibroblast growth factor-23, bone-specific alkaline phosphatase, trimeric N-terminal propeptide,32 tartrate-resistant acid phosphatase 5b and BMD was not investigated in the study. Fifth, the correlation between sclerostin and proximal femur and lumbar BMD reduction was investigated, while the correlation of sclerostin with total hip and radius BMD loss was not investigated.
ConclusionIn this study, serum sclerostin levels were similar in RTRs and healthy individuals. No correlation was found between sclerostin level and renal function. It was found that a significant correlation between high serum sclerostin and proximal femur BMD reduction in RTRs on low-dose steroid. Monitoring of serum sclerostin levels may be used as a valuable prognostic marker of FN BMD reduction and increased risk of bone fracture in RTRs at ≥1 year post-transplantation on low-dose steroid. Yet, there is a need for further multicenter studies with larger sample of patients since the results of the current studies in this respect are not uniform.
Statement of ethicsThe study protocol was approved by the local institutional review board.
Conflict of interestAuthors have nothing to declare.