The hypertriglyceridaemic waist (HTW) phenotype is defined for the general population. Chronic kidney disease (CKD) tends to bring on changes in body composition, is associated with higher comorbidity than the general population and, furthermore, shows reverse epidemiology with related prognostic variables like cholesterol and body mass index. Our objective was to identify cut-off points in the population with CKD and to analyse its relationship with cardiovascular risk (CVR).
MethodsWe included 2,271 CKD patients from the NEFRONA cohort. Triglyceride and waist cut-off points were selected through quintiles analysis and receiver operating characteristic (ROC) curves evaluation, using the presence of moderate to severe atherosclerosis score (AS 2-3) as outcome variable. Then, we analysed HTW prevalence and its association with other cardiovascular risk factors, and we measured the magnitude of its effect on AS 2-3 and cardiovascular event or death (CVEoD) by multivariate regression analysis.
ResultsWe selected the cut-off points: triglyceride concentrations ≥143 mg/dl with waist circumference values >102 cm in men and 94 cm in women (sensitivity 26%; specificity 87%). Specific HTW prevalence was 22.4%, without significative differences between CKD stages. The multivariate regression analysis shows specific HTW as an independent AS 2-3 (OR 1.61; 95% CI: 1.12-2.32, p = 0.011) and CVEoD (HR 3.08; 95% CI: 1.66-5.72, p = 0.000) risk factor. An interaction between phosphorus level and specific HTW was identified.
ConclusionsAdapting the HTW definition might improve specificity to assess cardiovascular risk in the population with CKD. It identifies an additional CVR in a population in which other screening methods have not proven to be useful, and it is easily clinically accessible. Its interaction with phosphorus levels suggests an association between HTW and bone-mineral metabolism regulation.
El fenotipo de cintura hipertrigliceridémica (FCH) se define para población general. La Enfermedad Renal Crónica (ERC) asocia cambios en la composición corporal, elevada comorbilidad y una epidemiología reversa en relación con el colesterol y el índice de masa corporal. Nuestro objetivo fue identificar los puntos de corte en población con ERC y analizar su relación con el riesgo cardiovascular (RCV).
MétodosIncluimos 2271 enfermos renales de la cohorte NEFRONA. Seleccionamos los puntos de corte de triglicéridos y cintura mediante análisis de quintiles y curvas ROC, utilizando presencia de enfermedad ateroesclerótica moderada-severa (EA2-3) como variable resultado. Analizamos la prevalencia del mismo y su asociación con otros factores de riesgo cardiovascular, incluimos análisis de regresión multivariable para medir la magnitud de su efecto frente a las variables EA2-3 y evento o muerte cardiovascular (EoMCV).
ResultadosSeleccionamos los puntos de corte: Triglicéridos ≥ 143 mg/dl con cintura > 102 cm en varones o 94 cm en mujeres (Sensibilidad 26%; Especificidad 87%). La prevalencia del FCH específico fue 22.4%, sin diferencias entre estadios de ERC. Asoció aumento de riesgo independiente frente a EA2-3 (OR 1.61; IC 95%: 1.12-2.32, p = 0.011) y EoMCV (HR 3.08; IC 95%: 1.66-5.72, p = 0.000). Identificamos una interacción entre FCH y fósforo.
ConclusionesAdaptar la definición del FCH en la población con ERC mejora su rendimiento diagnóstico. Identifica un RCV adicional en una población donde otros métodos de cribado no han mostrado utilidad, siendo de fácil acceso clínico. Su interacción con los niveles de fósforo podría reflejar un papel en la regulación del metabolismo óseo-mineral.
Cardiovascular disease is the most important cause of mortality in chronic kidney disease (CKD). Keith et al.1 reported that this population presented a greater risk of dying as a result of a cardiovascular event than of starting dialysis. In recent years, different research has focused on the search for new instruments that identify this risk as opposed to the traditional risk scales that are not valid in CKD. The carotid ultrasound and the ankle-brachial index (ABI) have proven their usefulness as subclinical atherosclerosis screening markers in these patients.2 Nevertheless, both explorations require specific technological equipment that is not always available in routine clinical practice.
On the other hand, access to the simultaneous measurement of triglyceride levels and the waist does not call for a major diagnostic effort and renders it possible to define the hypertriglyceridaemic waist phenotype (HWP). The HWP has proven its usefulness in the general population for the detection of the so-called atherogenic metabolic triad (AMT): hyperinsulinemia, hyperapolipoproteinaemia B and elevation of small dense low-density lipoprotein-cholesterol particles (sdLDL-C).3 It is also a useful screening tool for coronary disease and cardiovascular mortality.4,5
Zhe et al.6 identified a relationship between HWP and carotid intima-media thickness (CIMT) in the population with CKD. However, we know that this disease involves a greater comorbidity than that of the population in which the HWP was initially defined, with a complex pathophysiology for the development of cardiovascular disease and differential characteristics in relation to lipid metabolism and obesity, as well as a tendency to undergo changes in body composition. For this reason, our hypothesis poses the existence of different HWP cut-off points in subjects with CKD. We have not identified any studies that have evaluated this possibility before. This work will seek to identify the HWP cut-off points that best detect an increase in cardiovascular risk (CVR) in the population with CKD.
Material and methodsStudy design and participantsThe NEFRONA study was designed as a prospective and multicentre observational cohort study for the evaluation of the presence of subclinical atherosclerotic disease (AD) and an analysis of the predictive value of the carotid/femoral ultrasound study. It recruited patients between October 2010 and June 2012 from 81 hospitals or dialysis clinics representative of the whole of Spain. The study’s methodological details have already been published.7–9
Our analysis includes 2,271 patients with CKD. The inclusion criteria were being aged between 18 and 74 years, with an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2 (estimated with the CKD Epidemiology Collaboration creatinine equation [CDK-EPI] from 2009) or a urine albumin-to-creatinine ratio (ACR) of greater than 30 mg/g. The exclusion criteria were the same as for the NEFRONA cohort (prior cardiovascular event, active infection [HIV, tuberculosis], pregnancy, organ transplant, life expectancy <1 year), and patients on treatment with peritoneal dialysis were also excluded as there was no abdominal circumference record available for this subgroup.
Clinical, anthropometric and biochemical dataAt inclusion, the patients completed a questionnaire that included the clinical variables. All the anthropometric data were obtained by means of standardised methods.7 The waist was measured in centimetres at the height of the navel. The biochemical parameters were obtained by means of a routine blood test taken in a period of three months before or after the first vascular exploration. The NEFRONA study paid particular attention to sensitive parameters such as parathyroid hormone (PTHi) levels in subjects on dialysis, ultra-sensitive C-reactive protein (US-CRP) levels and vitamin 25(OH)D3.9
Evaluation of atherosclerosisDetailed technical information about the subclinical atherosclerosis evaluation methods has already been described.7 All the studies were conducted with standardised protocols by three travelling teams that included a nurse and a radiodiagnostic technician.
A carotid and femoral ultrasound study was performed, together with measurement of the ABI when the patients were included in the study. An AD score was defined according to the following criteria: AD0 (no atheromatosis), ABI > 0.9 and cIMT <90° percentile of the reference value; AD1 (mild), ABI 0.7-0.9 or cIMT ≥90° percentile; AD2 (moderate), carotid plaque with stenosis <75%; and AD3 (severe), ABI < 0.7 or carotid plaque with stenosis ≥75%.
Ethical principlesThe NEFRONA study was conducted in accordance with the criteria established in the Declaration of Helsinki and all the participants provided their informed consent. The study was approved by the Research Ethics Committee of Aragon.
Statistical methodsThe data are presented by means of medians ± interquartile ranges for continuous variables and as frequencies or percentages for categorical variables. The differences between medians were evaluated by the Mann Whitney Test since the majority of the variables followed a different-from-normal distribution. The categoric variables were compared by means of Pearson's chi-squared test. The Bonferroni correction was applied for the comparisons between columns in multi-categorical variables.
Different statistical methods were used to select the specific HWP cut-off points of patients with CKD. Firstly, a quintile analysis. Moreover, an ROC curve analysis was performed and Youden’s J statistic was calculated as a measure of optimisation of the cut-off points.
The relationship between the HWP and the AD2-3 was assessed by means of a multivariate logistic regression. In addition, we evaluated the magnitude of the effect of this phenotype with regard to the development of a cardiovascular event or mortality (CVEoM) in 48 months of follow-up with a multivariable Cox regression analysis. The possible confounding factors selected were variables with a significant association in the stratified Mantel-Haenszel analysis and in the univariate analysis, as well as potential confounding variables described in the literature. We also used a forward stepwise procedure to develop multivariable regression models, including the variables that presented a significant contribution in the identification of AD2-3 or CVEoM in accordance with the likelihood-ratio test. The variables without significance that modified the value of the coefficient (®) of the HWP by more than 10% when they were removed from the model were also included. We also performed a search for possible interactions with the HWP variable in both models. A p-value <0.05 was regarded as significant.
ResultsDemographic and clinical characteristics2,221 subjects were included (80 CKD G1-2 and A2-3. 888 CKD G3a-b. 852 CKD G4-5 and 451 on haemodialysis). 61.7% were men with a median age of 62 years. 25.8% were diabetics, 89.3% were hypertensive and 44.2% were non-smokers. The prevalence of dyslipidaemia was 65.3% and the proportion of subjects receiving lipid-lowering treatment was 63.4%. 1,825 patients were evaluated by means of carotid/femoral ultrasound (15.5% AD0, 13.2% AD1, 65.9% AD2 and 5.3% AD3).
We selected the presence of AD ≥2 as an outcome variable to establish the cut-off points for triglycerides and waist because these stages, and not the lower ones, were statistically significantly associated with the CVEoM combined variable (p <0.05 by means of the Bonferroni correction).
Selection of the cut-off pointsWe studied each diagnostic variable separately and in different sample subgroups. On the one hand, we evaluated triglycerides in the entire group of patients with CKD, and on the other hand only in the subgroup that was not receiving lipid-lowering drug treatment. The waist variable was evaluated separately in men and women.
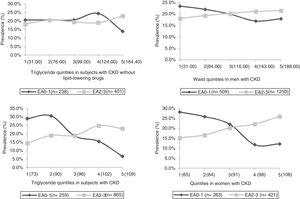
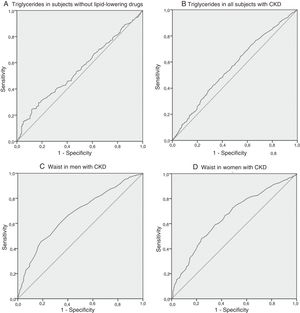
The quintile and ROC curve analysis is shown in Figs. 1 and 2. For the triglycerides variable, we selected levels above 143 mg/dl (1.6 mmol/L) on the basis of two aspects: first, subjects without lipid-lowering drugs that presented a higher optimal cut-off point are less representative of the population with CKD due to the high prevalence of dyslipidaemia in this population. Secondly, selecting the value of 143 mg/dl in this subgroup did not yield an important variation in sensibility/sensitivity versus the outcome variable compared to the cut-off point associated with the 5th quintile and the maximum Youden’s J statistic (164.5 mg/dL or 1.9 mmol/L). For the waist variable, we selected the cut-off points of 102 cm in men and 94 cm in women, mainly as a function of the maximum Youden’s J statistic and disregarding decimal values for the sake of greater simplicity.
The HWP defined specifically in subjects with CKD by these cut-off points was associated with a sensitivity of 26%, a specificity of 87%, a positive predictive value of 83%, a negative predictive value of 32%, a positive likelihood ratio of 1.9 and a negative likelihood ratio of 0.85 for the detection of moderate or severe forms of AD. Prevalence of HWP
The frequency of subjects with specific HWP was 22.4% (486/2170). We observed an age-associated increase in the frequency, reaching a maximum value in the subgroup between 63 and 68 years (30.3%), which in turn was the only one in which we identified gender-related differences (26.1% in men and 37.9% in women, p < 0.05 with Bonferroni analysis). Prevalence tends to diminish in the group of subjects on haemodialysis (19.5% on haemodialysis vs. 21.5% in CKD G1-3b and 24.7% in G4-5, p = 0.147), albeit without reaching statistical significance, unlike what we observed with other obesity-related variables such as body mass index or metabolic syndrome, as defined by the criteria of the International Diabetes Federation.
HWP and cardiovascular riskWe evaluated the association of HWP with other CVR factors by means of the definition established in the general and the specific population. HWP was associated with higher levels of blood pressure, pulse pressure, atherogenic index, non-HDL-cholesterol, apolipoprotein B, insulin resistance index, us-CRP, together with lower levels of vitamin 25OHD3, according to both definitions. However, only the specific definition was significantly associated with the presence of CVEoM (Table 1). We applied a multivariable regression analysis (Table 2) and observed that the magnitude of the effect of the latter continues to be significant after adjusting the models for the main confounding variables identified for both the AD2-3 variable (OR 1.61; 95% CI: 1.12-2.32, p = 0.011 vs. OR 1.09; 95% CI: 0.80-1.50, p = 0.580) and for the CVEoM combined variable (HR 3.08; 95% CI: 1.66-5.72, p = 0.000 vs. HR 1.08; 95% CI 0.71-1.64, p = 0.728). One striking finding was the identification of an interaction between the specific HWP and the levels of phosphorus with regard to the CVEoM variable. The multivariable regression analysis stratified by phosphorus levels showed that this association remained significant only in subjects with levels of phosphorus below 3.5 mg/dl (HR 2.80; 95% CI: 1.59-4.92, p = 0.000). On extending the stratification by CKD stages, the interaction only remained significant in the G4-5 stage subgroup (Table 3).
DiscussionWith regard to the cut-off points selected to define HWP in the Europids population (triglycerides above 177 mg/dl in men or 133 mg/dl in women, together with a waist greater than 90 cm in men or 85 cm in women),3,10 in this work we observed that levels above 143 mg/dl combined with a waist greater than 102 cm in men or 94 cm in women render it possible to optimise this tool’s diagnostic performance in the population with CKD. This variation could have several explanations. First of all, unlike the general population, where the cut-off points were selected by identifying non-traditional CVR factors (those that define AMT), we used the presence of a subclinical, but already established, atherosclerotic lesion as an outcome variable. Moreover, our kidney patient cohort was older and presented greater comorbidity compared to that of the original studies (median age 43.1 years, non-obese, with no treatment for coronary disease, diabetes, dyslipidaemia or endocrine disorders).3,10
The prevalence of HWP was considerable, irrespective of the definition used. The specific definition shows an age-related increase, as has also been described previously in the population without CKD,11,12 with the greatest peak in post-menopausal women. Other authors have previously shown an increase in visceral fat in women with this condition, relating it to a reduction in serum levels of oestrogens and dehydroepiandrosterone sulphate.13,14
We identified an association between the presence of this phenotype and different CVR factors. Thus, as in studies in a population without CKD,3,10 we observed a significant association with the atherogenic metabolic triad, including the increase in apolipoprotein B levels and insulin resistance. Although we did not have a determination of sdLDL-C levels, we observed that they were associated with higher levels of non-HDL cholesterol, a parameter that presented a good correlation with it (r = 0.76).15 We also identified an association with elevated levels of us-CRP to limits deemed to constitute a greater CVR (>2 mg/L).16 Individuals with HWP presented higher systolic blood and pulse pressure levels, suggesting greater arterial stiffness, although we did not detect any differences in vascular calcification as assessed by the presence of an ABI > 1.2. Similar to what has been described in obese subjects, the HWP was associated with a reduction in levels of 25OHD.3. Taking into account the poorer prognostic outcomes associated with levels <30 ng/dL in patients with CKD,17 we wondered whether screening and supplementing could be particularly beneficial in these patients.
The regression analysis showed an independent association between specific HWP and the presence of AD2-3. In relation to the variables selected to adjust the model, it should be emphasised that some of them were selected from the literature review, including previous publications with the NEFRONA cohort.9,18–21 The aforementioned work involved a noteworthy assessment of risk factors associated with the presence of atheroma plaque, pathological ABI or the progression of AD in patients with CKD. On this basis, we selected variables such as levels of phosphorus associated with greater CVR according to gender (above 3.5 mg/dl in men and 5 mg/dl in women)20 or the significant effect of the interaction between the levels of ferritin and age.21
Similarly, the specific HWP behaved as an independent risk factor for the development of CVEoM. However, we observed that this effect depended on the interaction with phosphorus serum levels and was only significant in patients with phosphorus below a 3.5 mg/dl at the beginning of the study. In the stratified analysis by CKD stages, the interaction only remained significant in the G4-5 stages. Garagarza et al.22 showed an association between hypophosphoremia and increased mortality in advanced forms of CKD, stating that the presence of hypoalbuminemia and hypovolaemia acted as possible confounding variables in these patients. In our work, we were unable to evaluate this information at the end of follow-up, although initial albumin values did not modify the outcomes obtained. Moreover, the possible role of the adipokines in these associations must be considered. Thus, in the population with CKD, the reduction in GF is described as the main determinant of elevated levels of adiponectin, which increase logarithmically as of stage 4, whereas body mass index is the main determinant of elevated leptin.23 By both routes, patients in G4-5 stages with HWP could present an elevation of both adipokines. More recently, the influence of adipose tissue in bone and mineral metabolism regulation has been described. In obese patients, the adipose tissue increases the release of factors associated with the stimulation of FGF-23, such as interleukin-6, tumour necrosis factor alpha and leptin. Moreover, experimental studies in transgenic mouse models with over-expression of adiponectin show how a surplus of phosphorus in the diet is associated with elevated FGF-23 levels and phosphorus excretion. It is no coincidence that FGF-23 is related to the development of structural cardiopathy and cardiovascular disease in the population with CKD.24–26 The association between HWP and CVEoM identified in subjects with lower phosphorus could be related to these physiopathogenic mechanisms. However, in our analysis we lacked sufficient information to be able to confirm this hypothesis.
The multivariable regression analysis showed a significant phenotype effect which also extended to the population on haemodialysis. In this same line, Postorino et al.27 studied patients on haemodialysis, describing a progressive increase in cardiovascular mortality and for all causes from fixed surpluses of triglycerides of 50 mg/dl in patients with a waist greater than 95 cm, as well as a reduction of the same as triglycerides increase in patients with lower waist limits. Therefore, our results, together with these findings, point to the need to consider a specific definition in the population on haemodialysis.
The main strong points of the study are those of the NEFRONA cohort, with a population sample that was representative of the entire Spanish territory, as well as information on a broad number of variables that enabled us to perform a powerful search and subsequently adjust for numerous confounding factors. Similarly, the NEFRONA Project featured a rigorous methodological design.7–9 Finally, attention should be drawn to the absence of cardiovascular events prior to the recruitment of the patients in the study, allowing us to identify cut-off points that could be useful in the detection of increased CVR in the subclinical stages.
This work also presents certain limitations. The first one is that we used data obtained transversely to infer a longitudinal relationship. Besides the models that we selected, other possible confounding variables could be involved, so other prospective studies that establish a more precise casual relationship would be called for. Secondly, the study needs to be completed with an external validation analysis. Thirdly, the group of subjects in the G1-2 and A > 1 stage was small, although we sought to control this bias by applying the Bonferroni correction to the analyses. Finally, in view of the absence of kidney transplant patients in the cohort and the added exclusion of patients on peritoneal dialysis due to the absence of a waist record, our results cannot be main-streamed to either of these two subpopulations. In this regard, there is work currently ongoing that establishes a suitable waist measurement technique in the population on peritoneal dialysis and relates this treatment to a progressive increase in this measurement, in turn independently associated with the reduction in adiponectin levels.28,29 At the same time, several publications relate inflammatory status, risk of diabetes and kidney transplant cardiovascular prognosis to abdominal circumference and other visceral obesity markers.30–32 For all these reasons, it would be very interesting to be able to study HWP in both populations.
In conclusion, adjusting the limits of the definition of HWP would appear to be necessary in the CKD population. The specific HWP of this population constitutes a simple tool that can be useful in consultations to identify a group of kidney patients with an additional CVR, and this also includes the population on haemodialysis. Our results arouse interest in the development of studies to evaluate the role of visceral obesity in the regulation of bone mineral metabolism in patients with CKD, as well as intervention design studies to evaluate possible interventions aimed at improving the cardiovascular prognosis of these patients.
HighlightsThe cut-off points for defining HWP in the population with CKD presenting the highest diagnostic performance were triglyceride levels >143 mg/dl and waist >102 cm in men and >94 cm in women.
The differences observed with regard to the cut-off points described in the literature could be accounted for by the greater age and comorbidity of the population with CKD, as well as the use of a different diagnostic variable (subclinical, but established, atherosclerotic disease, compared to atherosclerotic disease risk factors: atherogenic metabolic triad).
The prevalence of HWP in CKD is considerable, and also in patients on haemodialysis.
The interaction identified between HWP and phosphorus levels with regard to the development of CVEoM arouses interest in extending the study of the relationship between this phenotype and bone and mineral metabolism regulation.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank all the investigators involved in the NEFRONA study and its scientific committee for giving us the opportunity to take part in this project.
Please cite this article as: Bielsa-Gracia S, Miguel Lou L, Antonio Gimeno J, Gracia-García O, López-Alejaldre I, Fernández E. Fenotipo de Cintura Hipertrigliceridémica en la población con Enfermedad Renal Crónica. Cohorte NEFRONA. Nefrologia. 2020;40:514–521.