Introducción: La obesidad aumenta el riesgo de proteinuria e insuficiencia renal crónica, y acelera la progresión de enfermedades renales. En los pacientes obesos existe un aumento de la actividad del sistema renina-angiotensina-aldosterona (SRAA) y de los niveles de aldosterona. Ningún estudio ha comparado la eficacia de las diferentes estrategias antiproteinúricas actualmente disponibles (inhibidores de la enzima convertidora de la angiotensina [IECA], antagonistas de los receptores de la angiotensina [ARA], antagonistas de la aldosterona) en pacientes obesos con nefropatías proteinúricas. Métodos: Es un estudio prospectivo y aleatorizado, realizado en un único centro. Fueron seleccionados doce pacientes obesos (índice de masa corporal >30 kg/m2), con proteinuria >0,5 g/24 h, de nuestras consultas de Nefrología. Los pacientes fueron tratados consecutivamente durante seis semanas y en orden aleatorio con un IECA (lisinopril 20 mg/día), una terapia combinada con IECA más ARA (lisinopril 10 mg/día más candesartán 16 mg/día) y eplerenona (25 mg/día). Se estableció un período de lavado de seis semanas entre los diferentes períodos de tratamiento. El objetivo principal del estudio fue el cambio en la proteinuria de 24 h al final de cada período de tratamiento y el número de pacientes que mostraban una reducción de la proteinuria superior al 25% con respecto al valor basal. Resultados: La reducción de la proteinuria obtenida por lisinopril (11,3 ± 34,8%) no fue estadísticamente significativa con respecto al valor basal, mientras que la reducción con lisinopril y candesartán (26,9 ± 30,6%) y eplerenona (28,4 ± 31,6%) mostró una diferencia estadísticamente significativa frente a sus valores basales (comparación intragrupo) y frente al grupo de lisinopril (comparación entre grupos). El número de pacientes que mostraron una reducción mayor al 25% de la proteinuria fue significativamente mayor con eplerenona (67%) y lisinopril + candesartán (67%) que con lisinopril (25%). Conclusiones: La monoterapia con antagonistas de la aldosterona (eplerenona) y la terapia de combinación con IECA + ARA fueron más efectivos que los IECA en monoterapia para reducir la proteinuria en pacientes obesos con diferentes tipos de nefropatías crónicas proteinúricas.
INTRODUCTION
Obesity is a known cause of proteinuria and progressive renal damage.1-3 Recent studies show that the glomerulopathy associated with obesity is an increasingly diagnosed entity and has an increasing incidence.3,4 Furthermore, the role played by obesity in the progression of various renal diseases has been demonstrated.5-7 The amount of proteinuria is a significant risk factor in the progression of renal disease in subjects with diabetic and non-diabetic nephropathies, and any treatment which allows a reduction in the quantity of proteinuria will result in a favourable renoprotective influence on the long-term development of renal function.8,9 Among these treatment options, blockage of the RAAS in its various forms, ACEIs, ARAs, the combination of ACEIs and ARAs, and more recently, aldosterone and renin antagonists, represent the most effective antiproteinuric measures.10-13 In view of this, the role of obesity on the development of renal diseases and the epidemic proportions which obesity has reached in developed societies, the response of obese patients with proteinuric nephropathies to the various strategies which block the RAAS is a matter of extraordinary clinical significance, bearing in mind that the activity of the RAAS is increased by obesity.14,15 Recent studies suggest that obese patients show an increase in the synthesis of aldosterone, which could play an important role in the various complications associated with obesity, including renal damage.16,17 Experimental investigations undertaken on obese animals have demonstrated that the use of aldosterone antagonists drastically reduces the progression of renal lesions.18 These investigations suggest that obese patients with proteinuric nephropathies could have a better antiproteinuric response to aldosterone antagonists than to traditional RAAS blockage with ACEI or ARA. However, there is no clinical study that has specifically examined this matter. In order to try to demonstrate this hypothesis, we designed a prospective, randomised study, to compare the antiproteinuric efficacy of an ACEI (lisinopril), the combination of an ACEI and an ARA (candesartan plus lisinopril), and an aldosterone antagonist (eplerenone) in obese patients with proteinuria.
MATERIAL AND METHOD
Patients
The clinical study was approved by the clinical trials committee of our hospital and a signature of informed consent was requested from each patient prior to their inclusion in the study. The patients were selected in the Nephrology unit of our hospital. All the patients fulfilled the following criteria: a proteinuria level greater than 0.5g/24 hr in at least three consecutive tests in a period of six months prior to the study; obesity, defined as a body mass index (BMI) greater than 30kg/m2; and stable renal function with glomerular filtration rate (GFR) > 15ml/min/1.73m2. Patients undergoing rapid deterioration of renal function,with poor control of mean blood pressure (MAP > 100mmHg), patients who required more than three different anti-hypertensive drugs, with an unstable clinical condition, and patients receiving treatment with immunosuppressants or NSAIDs, were excluded. Both diabetic and non-diabetic patients were included in the study.
Design
The patients selected in this study belong to the same hospital centre; the study was prospective and randomised. Those patients taking RAAS blocking drugs were informed that it was necessary to discontinue their use for at least six weeks prior to the beginning of the study. All other anti-hypertensive drugs, including diuretics, were kept at the same dosage throughout the study. In addition, another series of drugs was kept without changes (statins, hypoglycaemic drugs and insulin in diabetic patients). Doxazosin was administered in some patients during the six-week period prior to randomisation and during the washout periods for the control of blood pressure. Patients kept their usual diet. After randomisation, patients were included consecutively for a period of six weeks in treatment with lisinopril (20mg once per day), lisinopril (10mg once per day) plus candesartan (16mg once per day) and eplerenone (25mg once per day) in random order. Randomisation was carried out by means of envelopes containing the order of treatment which the patient was to receive. The resulting order was ACEI, ACEI plus ARA, eplerenone in four patients, ACEI plus ARA, eplerenone, ACEI in another four, and eplerenone, ACEI, ACEI plus ARA in the remaining four. The study had an open design. A six-week washout period was established between the three different periods of treatment. The study medication was administered during the morning.
Clinical and laboratory parameters
At baseline and end of each period of treatment the various clinical and biochemical data were collected. A general physical examination was undertaken, including measurements of body mass index (weight in kilograms divided by height squared, in metres) and of the waist circunference. Blood pressure (AP) was measured after five minutes¿ rest with the patient sitting, using an automatic device. The average of [the] three readings was recorded. The mean blood pressure (MAP) was calculated as the sum of one third of the systolic blood pressure (SBP) and two thirds of the diastolic blood pressure (DBP). The tests carried out included complete blood count, serum creatinine, glucose, sodium, potassium, uric acid, calcium, phosphorous, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, total proteins, albumin, bilirubin, hepatic transaminases, C-reactive protein and glycosylated haemoglobin in diabetic patients. The blood samples for plasma renin and aldosterone activity were also taken at each visit, following 30 minutes¿ rest in the dorsal decubitus position. The day before each visit patients collected urine samples over a period of 24 hours for urine tests for proteinuria, sodium, potassium and excretion of creatinine. In addition, a sample of first morning urine was taken in order to carry out the protein to creatinine ratio. A standard autoanalyzer was used for measuring the biochemical parameters. Activity of plasma renin was measured by RIA, which detects the quantity of angiotensin I produced per hour, with excess angiotensinogen present (nanograms of angiotensin I produced per millilitre of plasma per hour). Aldosterone was measured using an RIA kit.
Outcomes measures
The primary outcomes of the study was the change in 24 hr proteinuria at the end of each period of treatment. In addition, the number of patients showing a reduction in proteinuria higher than 25% with respect to the baseline value of each period of treatment was analysed. The secondary outcomes were the changes in renal function (estimated by serum creatinine and GF) and in serum potassium levels.
Sample size
Previous studies6,19 demonstrated that the addition of antialdosterone drugs (eplerenone or spironolactone) to other RAAS-blocking agents resulted in a 25-50% further reduction of baseline proteinuria. In a pilot study undertaken by our group (details not published), it was found that the reduction of proteinuria in obese patients was 35% greater with antialdosteronic agents than with ACEIs or ARAs. With the confidence level set at 95%, it was estimated that 11 patients were required in order to adhere to the design of this study. Finally, 12 patients were selected to undertake the study.
Statistical analysis
The results are represented as average ± standard deviation and with the range between the minimum and maximum values. In order to establish comparisons between the groups with respect to the baseline values, Student¿s t-test and the Mann-Whitney test were used. Student¿s t-test or the Wilcoxon test were used for comparisons within the groups. For comparisons between groups, the Kruskal-Wallis test and the Mann-Whitney test were used. Correlations were undertaken by means of the Pearson test. Values with p < 0.05 were considered significant. Statistical analysis was carried out with the SPSS programme, version 13.0 (SPSS Inc, Chicago, IL).
RESULTS
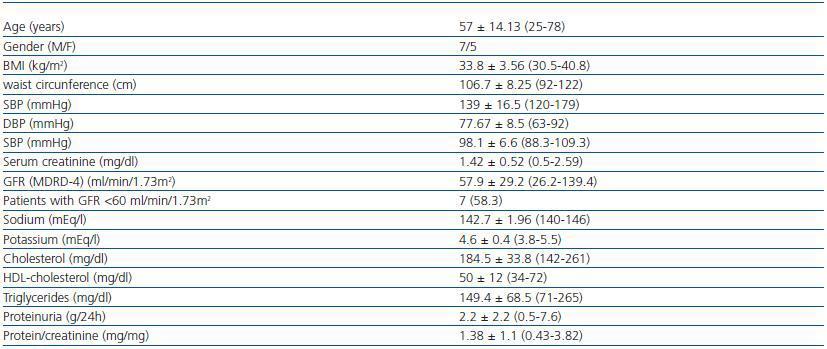
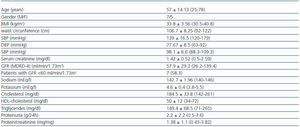
Twelve patients were included (seven men, five women), all of whom were Caucasian. In table 1 the clinical characteristics of the patients studied are summarised. Seven patients (58%) had a GF less than 60 ml/min/1.73 m2. The diagnoses were diabetic nephropathy (5), focal segmental glomerulosclerosis associated with obesity (3), IgA nephropathy (2) and nephroangiosclerosis (2).
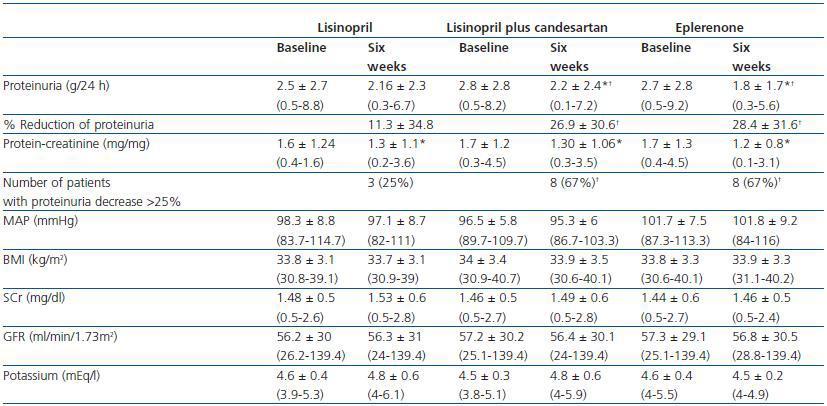
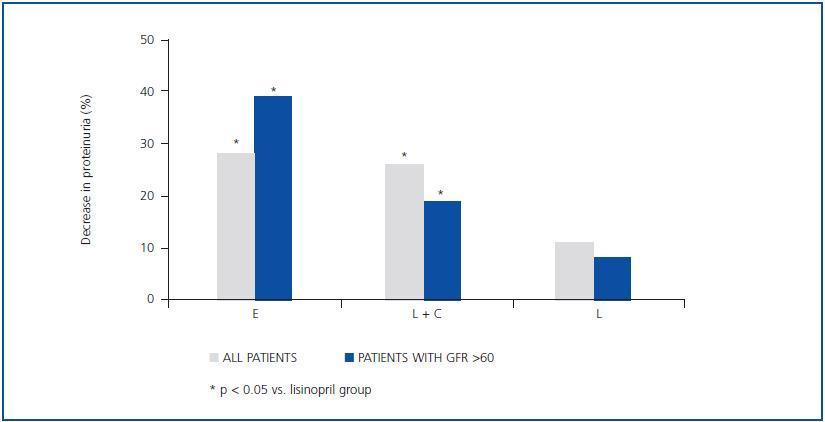
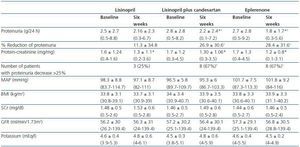
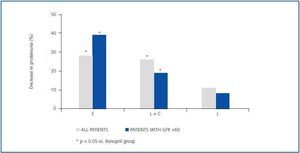
As shown in table 2 and figure 1, both eplerenone and the combination of lisinopril and candesartan obtained a significantly higher level of reduction of proteinuria than lisinopril. The observed reduction of proteinuria brought about by lisinopril (11.3 ± 34.8%) was not statistically significant with respect to the baseline value (p = 0.158), while those with the combination of lisinopril and candesartan (26.9 ± 30.6%), and eplerenone (28.4 ± 31.6%) showed a statistically significant difference with respect to the baseline values (comparison within the group p = 0.045 and p = 0.034, respectively) and to the lisinopril group (comparison between groups, p = 0.041 and p = 0.034, respectively).
The influence of the three treatments on the protein-creatinine ratio demonstrated a trend similar to that obtained with the values of 24-hour proteinuria, which indicates that the results were not influenced by possible errors in the collection of the urine sample. The reduction in the ratio obtained by lisinopril plus candesartan and eplerenone (26.3 ± 21.6 and 27.2 ± 22.5%, respectively) was greater than that obtained by lisinopril (17.3 ± 19.6%), although these differences were not statistically significant in comparison between groups (table 2). The number of patients showing a reduction in proteinuria greater than 25% with respect to the baseline values was significantly higher with eplerenone (n = 8, 67%) and with lisinopril plus candesartan (n = 8, 67%) than with lisinopril (n = 3, 25%, p = 0.026 with respect to the other groups), as shown in table 2.
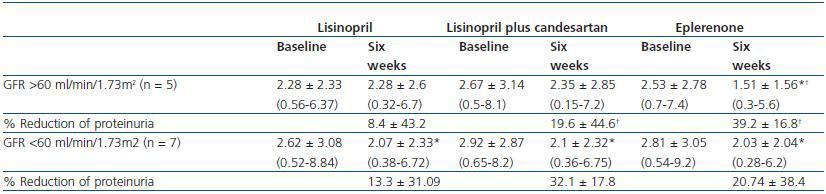
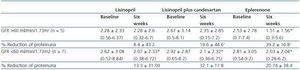
As indicated in table 3 and in figure 1, the antiproteinuric efficacy of eplerenone was more marked in patients with conserved renal function (GFR > 60ml/min/1.73m2). In this group of patients, the proteinuria decreased significantly by 39 ± 16% with respect to the baseline values after six weeks under treatment with eplerenone (p = 0.043), while in patients with a GFR < 60ml/min/1.73m2 it was 20 ± 38% with respect to the baseline values. This trend (a higher antiproteinuric response in patients with conserved renal function) was not observed in the other two treatment groups (monotherapy with lisinopril and the combination of lisinopril plus candesartan).
The reduction of proteinuria was independent of the changes in AP, body weight or renal function. No significant correlations were found between the reduction of proteinuria and baseline proteinuria, the activity of plasmatic renin or aldosterone.
Modifications in arterial pressure, body mass index, renal function and serum potassium
As shown in Table 2, there were no significant changes in AP, BMI, serum creatinine or GFR during the three different periods of treatment. This stability was also observed among the patients with GFR < 60ml/min/1.73m2 and the diabetic patients when they were analysed separately. The level of serum potassium was shown to undergo a slight increase, although not a significant one, in the three groups; no differences were found between the various treatment groups. The number of patients in which serum potassium was above 5.5mEq/l after treatment was 2/12 (16%) with lisinopril and 2/12 (16%) with lisinopril plus candesartan, while none of the patients treated with eplerenone reached this level of potassium. All the patients in which serum potassium exceeded 5.5mEq/l had a baseline GFR < 60ml/min/1.73m2. In the group of patients with GFR < 60ml/min/1.73m2 (n = 7), the increase of serum potassium was 4.8 ± 0.4 to 5 ± 0.6mEq/l with lisinopril (p = 0.398), 4.6 ± 0.3 to 5 ± 0.6mEq/l with lisinopril plus candesartan (p = 0.043 with respect to the baseline value) and 4.8 ± 0.4 to 4.7 ± 0.2 with eplerenone (p = 0.735). The changes in serum potassium of the diabetic patients were similar to those of the non-diabetic patients.
Renin, aldosterone and other laboratory values
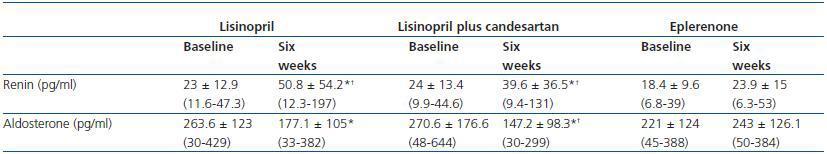
An increase in plasmatic renin was observed in patients treated with lisinopril and lisinopril plus candesartan, while renin remained stable with eplerenone. Similarly, serum aldosterone showed a significant reduction with lisinopril and with the combination of lisinopril plus candesartan, in comparison with eplerenone (table 4).
There were no significant changes in the values of glucose, sodium, uric acid, calcium, phosphorous, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, total proteins, albumin, bilirubin, hepatic transaminases, C-reactive protein or glycosylated haemoglobin.
DISCUSSION
The blockage of the RAAS, whether using ACEI or ARA in monotherapy or in combination, and more recently, with renin and aldosterone antagonists, represents the most effective treatment in the reduction of proteinuria in any chronic proteinuric nephropathy.10-13 The renoprotection obtained by these drugs is closely related to its antiproteinuric effect. However, experimental and clinical studies have demonstrated that RAAS activity is increased by obesity and that the adipose tissue, especially the visceral adipose tissue, produces all the components of the RAAS.14,15 On the other hand, obese patients show high plasmatic levels of aldosterone18 and recent studies have demonstrated that visceral adipocytes may secrete certain factors which increase the production of aldosterone by the adrenal glands, by means other than the classic renin-angiotensin routes.19 Oxidised fatty acids, commonly high in obese people, may also increase the synthesis of aldosterone.20 This group of data may suggest a greater antiproteinuric effect of the aldosterone antagonists in obese patients, due to the hyperaldosteronism associated with obesity. The results of our study support this hypothesis. We found that the reduction of proteinuria attained after six weeks of treatment with lisinopril was somewhat modest (11.3 ± 34.8%). The reductions obtained by the aldosterone antagonists, eplerenone (28.4 ± 31.6%) and by the combination of ACEI plus ARA (lisinopril plus candesartan) (26.9 ± 30.6%) were significantly more effective, and this higher level of response cannot be explained solely by the differences in the blood pressure values in the treatment groups. The number of patients who attained reductions in proteinuria higher than 25% with respect to the baseline was significantly higher with eplerenone and with the combined treatment than with monotherapy with lisinopril.
The discovery that eplerenone is more effective than monotherapy with ACEI in the reduction of proteinuria in obese patients is interesting, especially considering that we used a relatively low dosage (25mg/day). Epstein et al. demonstrated that the coadministration of eplerenone (dosage of 50-100mg) with an ACEI, in comparison with an ACEI in monotherapy, was more effective in the reduction of albuminuria in diabetic patients, without significant increases in potassium values being observed.21 The reduction of proteinuria brought about with a dosage of 50mg eplerenone (41%) was not significantly greater when the dosage was doubled to 100mg/day (48%). Curiously, although this study was not designed specifically for obese patients, the average BMI of the patients included was higher than 30kg/m2.21 The dosage of 50mg/day of eplerenone is the commonly used dosage in patients with heart failure, a clinical condition in which aldosterone antagonists have also shown to have a beneficial effect.22 As a result, it is possible that higher dosages of eplerenone (50mg/day) may increase the antiproteinuric effect observed in our study. The reason for using low dosages of eplerenone (25mg/day) in our study was that over half of the patients had kidney disease (GFR < 60ml/min/1.73m2) and, therefore, the risk of hyperkalemia could theoretically be higher in these patients. However, as shown in Table 2, serum potassium remained stable in the three treatment groups. Only two patients (16%) treated with lisinopril and lisinopril plus candesartan showed serum potassium level higher than 5.5mEq/l, while none of the patients treated with eplerenone showed these levels of potassium. These results are consistent with previous studies which show a low incidence of hyperkalemia in patients with kidney disease under treatment with aldosterone inhibitors.12,21 However, it must be emphasised that the results of our study are short term (six weeks of treatment); further long term studies are necessary to confirm the safety of aldosterone antagonists in patients with different nephropathies, and in particular in those patients with chronic kidney disease. In this respect, it is noteworthy that in our study eplerenone was particularly effective in patients with conserved renal function (GFR > 60ml/min/1.73m2): the antiproteinuric effect in these patients was almost doubled with respect to the patients who had a GFR < 60 (39 ± 16% vs. 20 ± 38%), as shown in Table 3. Various clinical studies published in recent years have highlighted the renoprotective and antiproteinuric efficacy of aldosterone inhibitors, both spironolactone and eplerenone. The majority of these studies were designed to analyse the antiproteinuric effect of aldosterone blockage when added to treatment with an ACEI or an ARA,23-29 and all of the studies demonstrated a significant reduction of proteinuria with this treatment option. However, few studies have been carried out which compare the antiproteinuric efficacy of aldosterone antagonists against ACEIs or ARAs. Epstein et al., in a prospective, randomised study of a large number of type-2 diabetic patients, demonstrated that eplerenone (200mg/day) had a greater antiproteinuric effect than enalapril 40mg/day.30 Rachmani et al. demonstrated that spironolactone (100mg/day) was more effective than cilazapril (5mg/day) in reducing albuminuria in type-2 diabetic women.31 In both studies, the combination of spironolactone or eplerenone with the ACEI was more effective than any of the two drugs taken separately.30,31 Our results, obtained in obese patients with diabetic and non-diabetic proteinuric nephropathy are consistent with the results obtained in these previous studies. In addition, we found that the ACEI-ARA combination had a significantly higher antiproteinuric effect than monotherapy with ACEI, and that its efficacy was similar to that of treatment with eplerenone. Various studies and meta-analyses have suggested that the antiproteinuric response with combined treatment of ACEI plus ARA is higher than that found with monotherapy of ACEI or ARA in higher dosages, with no differences in the control of arterial pressure which could interfere in the results.11,32,33 However, it is necessary to highlight that recent studies have shown a higher incidence of adverse effects (duplication of serum creatinine, dialysis) and mortality with the combination of an ACEI plus an ARA than with monotherapy with these drugs, although it was confirmed that the highest antiproteinuric efficacy was with the combined treatment.34
In the context of the limitations of our study, we must indicate that the periods of treatment were only six weeks. This fact only allows us to suggest a possible renoprotective effect of the aldosterone antagonists, associated with a higher antiproteinuric efficacy. With respect to the duration of the study, it is important to consider that, although this time is insufficient to verify the safety profile of the various pharmacological groups (eplerenone, ACEI or ACEI plus ARA), no hyperkalemias or changes in the glomerular filtration were observed during the six weeks of treatment. Obviously, it is necessary to carry out prospective studies with a longer follow-up period, which would allow the renoprotective effect of these drugs to be demonstrated. It must be borne in mind that both ACEIs and ARAs in monotherapy have shown a clear renoprotective effect in significant prospective studies in patients with diabetic and non-diabetic nephropathy.35-40
In summary, our study indicates that aldosterone antagonists represent a treatment option that could be of great interest for obese patients with proteinuric nephropathies, both diabetic and non-diabetic. These results would support the role of aldosterone in the pathogenesis of renal damage induced by obesity, as various recent experimental studies have shown. However, studies with a greater number of patients and a longer follow-up period are necessary, in order to conclusively evaluate the efficacy and safety of these drugs.
What is known about this issue?
- The amount of proteinuria is a risk factor in the progression of diabetic and non-diabetic chronic proteinuric nephropathies. The addition of aldosterone antagonists to regular treatment with ACEI or ARA significantly increases the reduction of proteinuria in these patients.
- Obese patients undergo an increase in the synthesis of aldosterone, which may play a fundamental role in the complications linked with obesity. Aldosterone antagonists may reduce the renal lesions observed in animal models of obesity.
What is the contribution of this study?
- Monotherapy with an aldosterone antagonist (eplerenone) is more effective than an ACEI in monotherapy (lisinopril) and of a similar efficacy to the combination of an ACEI plus ARB (lisinopril plus candesartan) in the reduction of proteinuria in obese patients with different types of proteinuric nephropathies.
- Eplerenone, like ACEI and the combination of ACEI plus ARB were well tolerated, and no significant hyperkalemias or deterioration of renal function were observed.
Table 1. Patients' clinical characteristics
Table 2. Changes in proteinuria, blood pressure, BMI, renal function and serum potassium during treatment
with lisinopril, lisinopril plus candesartan, and eplerenone
Figure 1.
Table 3. Changes in proteinuria during treatment with lisinopril, lisinopril plus candesartan, and eplerenone
in patients with a GFR higher or lower than 60ml/min/1.73m2
Table 4. Changes in the values of renin and aldosterone during treatment with lisinopril, lisinopril plus
candesartan, and eplerenone