La respuesta inmunitaria a la vacuna de la hepatitis B (HB) está impedida en los pacientes en hemodiálisis (HD), y la persistencia de la inmunidad, la eficacia de la revacunación y la periodicidad de la realización de controles serológicos no están bien definidas. Presentamos la experiencia de un protocolo de vacunación de la HB con tres dosis intramusculares de 40 μg de vacuna recombinante (Engerix®-B) en un grupo de 136 pacientes atendidos en una unidad de HD a lo largo de 18 años. Se realizaron controles anuales de anticuerpos anti-HB en todos los pacientes, y semestrales en 31; y se administraba anualmente una dosis doble de vacuna a los pacientes que no respondían o cuando los niveles de anticuerpos descendían por debajo de 10 UI/ml. Setenta y cuatro pacientes (54,4%) presentaron seroconversión, mientras que 62 pacientes no respondieron. La edad de los pacientes era superior en el grupo de no respondedores, pero no se observaron diferencias en el sexo ni en la etiología de la enfermedad renal. Un 32% de los pacientes respondedores perdió la memoria inmunológica al primer año de la vacunación, y tan sólo un 18% de los pacientes permaneció inmunizado a los seis años. El título de anticuerpos inmediatamente después de completar la vacunación fue predictor del mantenimiento de la memoria inmunológica: un 75% de los pacientes con títulos de anticuerpos >1.000 UI/ml mantuvo la seroprotección a los tres años en comparación con un 47% con títulos entre 100-999 (p = 0,08), y un 34% con títulos entre 11-99 (p = 0,02). La administración de dosis de refuerzo fue efectiva en un 24% de los pacientes no respondedores, y un 69% mantenía la respuesta inmunológica al final del primer año. Las dosis de refuerzo repetidas en pacientes no respondedores a una primera dosis consiguieron nuevas seroconversiones en un 19,6% de los pacientes. La práctica de controles semestrales podría haber permitido administrar dosis de recuerdo antes del período anual en un 16% de los pacientes respondedores. En conclusión, nuestros resultados demuestran que un protocolo de vacunación de la HB con un seguimiento serológico regular y dosis de refuerzo sucesivas consigue una aceptable seroprotección en los pacientes en hemodiálisis.
INTRODUCTION
In the 1960s and 70s, HB was a serious problem in the HD services. In the last decades, the rate of infection from the HB virus in this group of patients has markedly decreased due to improved study methods, stricter monitoring in blood banks, reduced transfusion requirements subsequent to the availability of erythropoietin, optimisation of haemodialysis in patients infected with the HB virus and to the widespread use of vaccination.1,2
The immunological response to the HB vaccine is related to the degree of kidney failure, with greater response in patients with predialysis chronic kidney disease,3 and therefore earlier vaccination in this stage of the kidney disease is advisable. On the contrary, in HD patients, response to the HB vaccine is quite variable and lower than in the general population,4,5 with a response even below 50% with the administering of three doses of vaccine6,7 and slightly higher when a four-dose course is administered.8 In addition to this, persistence in the immunological response is also lower, with a fast decrease in the protective antibody titres in many of these patients.4,5,9
The mechanisms responsible for this decrease in the immunological response are unknown. It has recently been proven that the low serological response to the HB virus in this population is closely linked to the deficit in specific-antigent dependent production of IL-2 by CD4+ Tcells.10
With the aim of improving response to the HB vaccine in the HD population, several alternatives have been proposed, such as increasing the administered vaccine dose,11 intradermal application,12 co-administration of zinc or several immunomodulators,4,5 or the more recent use of a new adjuvant, AS04, a derivative of bacterial lipopolysaccharides.13 These interventions have obtained variable results.14
Many of the studies published on the HB virus in haemodialysis patients are centred on the results of this population¿s seroconversion,8 but the information on immunity evolution of responding patients or those that have been revaccinated is much more limited.
The aims of this study were: a) to assess the initial response to the HB vaccine in haemodialysis patients, the factors that influence such response and its relationship with progressive antibody decay; b) to analyse the efficiency and usefulness of revaccination; and c) to assess if the biannual antibody level tests bring in additional information to that obtained in the annual tests.
PATIENTS AND METHOD
Retrospective study that analyses a vaccination protocol against HB carried out in an HD service from 1989 to 2002. One hundred and thirty six patients in the HD program with anti-Hbc (-) and anti-Hbs (-), with no evidence of previous infection or history of vaccinations, were vaccinated against HB at the start of renal substitutive treatment. The patients received three 40μg doses of recombinant vaccine (Engerix® -B, Glaxo Smith Kline Biologicals, Rixensart, Belgium), injected intramuscularly in the deltoids, at months 0, 1 and 6. Annual antibody tests were performed and an annual booster double dose of vaccine was administered to non-responding patients or in the case of responding patients that lost their antibody levels (< 10UI/ml). Patients were followed until 2007 or until their decease, change of treatment service or kidney transplant.
Response to the vaccine was analysed in all patients, together with the factors influencing immunization and the efficiency of revaccination in patients who did not respond to the initial vaccination. Maintenance of the level of antibodies after the first positive revaccination was also assessed.
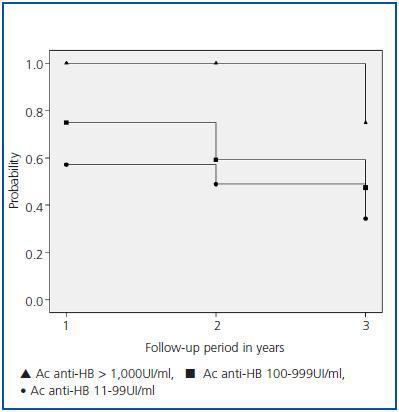
A subgroup of 63 patients with a three year follow-up after the first year of vaccination was analysed for the probability of maintaining the immunological response according to the antibody titres reached during the first annual control. Patients were classified into three groups: group I, patients with antibodies > 1,000UI/ml; group II, patients with antibodies between 100 and 999UI/ml; and group III, patients with antibodies from 11 to 99UI/ml.
Finally, a subgroup of 31 patients was analysed to check if the biannual antibody level tests contributed any additional information to their annual tests.
The anti-Hbs antibodies were determined via microparticle enzyme immunoassay (MEIA). The lower limit of antibody detection was 2mUI/ml and the intra- and inter-assay variation coefficient was 4.4 and 4.8%, respectively. Patients with titres equal to or over 1,000UI/ml were considered as > 1,000UI/ml.
The anti-Hbs antibody titres > 10UI/ml were considered protective, while the lower titres were defined as loss of immunity.
The statistical analysis was performed using the SPSS 11.0 program. Results are expressed as mean + SD. The comparison between quantitative variables was carried out with Student¿s T-test and for the categorical variables the chi-square was used.
Duration of immunity according to the anti-Hbs antibodies was analysed using the Kaplan-Meier estimator.
Values p < 0.05 were considered significative.
RESULTS
Responding patients
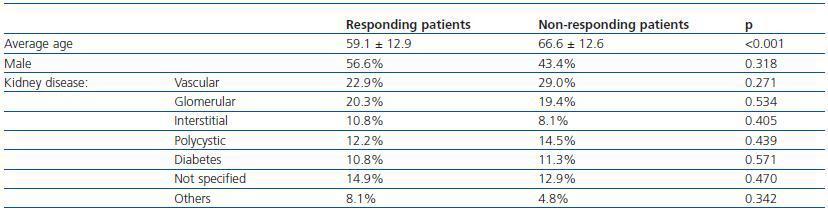
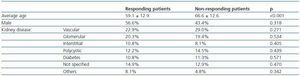
Seventy four patients (54.4%) developed immunity against the HB vaccine. Of these, 39 patients (52.7%) presented an antibody titre > 100UI/ml, a level which has been considered as the necessary threshold to maintain adequate seroprotection in HD patients. Response to the HB vaccine was related to age, the average non-responding age was higher than that of the responding patients. Conversely, the sex and aetiology of the chronic renal disease did not influence the response (table 1).
In successive annual tests a progressive decrease in the levels of antibodies was detected. In the first annual post-vaccination testing, 67.8% of the patients maintained protective levels, and six years later, only 17.8% of the patients maintained the immunological memory.
Non-responding patients
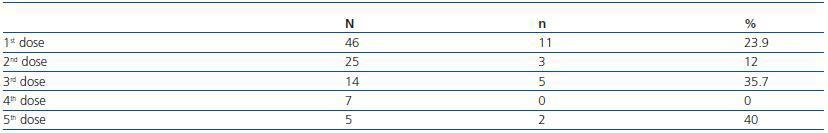
The 62 patients that did not respond to the three initial doses of vaccines were subject to an additional booster dose; although only 46 patients were evaluated at the end of the first years, since the remaining patients passed away, had transplants or were transferred to another service. The global efficiency for these doses was 21.6%: with the first dose, 23.9% of the patients seroconverted; with the second dose, 12%; and with the third, 37.7% (table 2). 42.8% of the seroconverted patients presented a level of anti-Hbs antibodies > 100UI/ml. The percentage of patients that maintained protecting levels of antibodies after the first booster dose was 69.2% in the first annual control.
Immunological response and antibody titre
In the 63-patient subgroup with a three year follow-up, 7 patients were included in group I, 28 in group II and 28 in group III. The average age of these groups was 55.5 (33-78), 62.8 (29-78) and 57.2 (26-75), respectively. The antibody titre in groups II and III was 55 (12-98) and 290 (100-978) UI/ml, respectively.
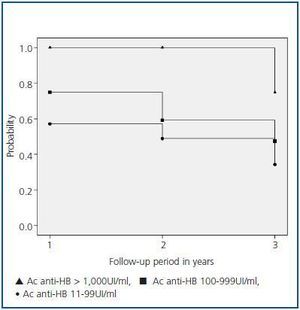
Figure 1 details the probability of patients remaining protected after vaccination. After follow-up, 75% of the patients in group I maintained seroprotection versus 47% in group II (p = 0.08) and 34% in group III (p = 0.02). Conversely, no significant differences were found between groups II and III.
Importance of the biannual antibody tests
Of the 31 patients that had biannual and annual antibody tests, the biannual tests supplied additional information. In four of the 25 responding patients, the levels of antibodies had become negative six months later. Therefore, these patients could have benefited from an earlier booster shot. Finally, seven non-responding patients to the annual testing presented protecting antibody levels in the biannual tests. This group of patients would have been considered non-responding to the vaccine if they had undergone only one annual test.
DISCUSSION
In this study, an HB vaccination protocol which ended in 2002 is presented, using the conventional guideline of three double doses of vaccine. Since 2003, this protocol was amended, upon completion of the study, increasing it to four double doses of vaccine,15,16 guideline which is currently being followed in our Service.
Despite the current controversy as to the effectiveness of the HB vaccine in HD patients, due to the reduced efficiency of the vaccine and the low incidence of infection by the HB virus, its use has become generalised in this population of patients.17 In our experience, 54.4% of the vaccinated patients developed immunity, a figure similar to that shown in other studies.8,18 Even in the protocols that include four double doses of vaccine in months 0, 1, 2 and 6, the seroconversion results obtained are not optimal either and oscillate between 58 and 80%.8,19 This insufficient response to the HB vaccine is related to deficiency in immunocompetence, characteristic of advanced chronic renal disease.4,5 Other modulating factors that negatively affect this response have been identified, such as age, diabetes, malnutrition, erythropoietin deficiency and suitability and duration of dialysis, among others.19,20 The lack of response of our patients was related to age, the percentage of responding patients decreasing the higher the patient age. In contrast, other factors, such as the presence of diabetes, did not influence the response in this population.
In patients who did not respond to the initial vaccination, revaccination was recommended with three additional vaccine doses, obtaining a response in 40-50% of the patients,8 although in some studies these were much lower.7 Intradermal injection has proven to be useful as a revaccination method in non-responding patients, although it poses technical problems in its administration.12 There is speculation that the non-responding patients to the initial vaccine could have developed cellular immunity without humoral immune response and that revaccination could be able to induce humoral immunity in this population.21
The guide to viral infections in haemodialysis by the Spanish Society of Nephrology (SEN)2 recommends the administration of a second vaccine guideline in patients who have not responded to vaccination (AcHbs < 10UI/L). If there is still no response, the patients are then definitively considered non-responding patients, and there is no evidence that non-responding patients with both guidelines applied have a higher rate of subsequent response. Our results show that the establishment of a booster dose administration protocol in patients without initial response allows for immunisation in a large number of patients. Thus, with this protocol, with the first dose, a quarter of the non-responding patients were immunised and the administration of new booster doses obtained new immunisations, even after five booster doses.
One third of our responding patients loss immunological memory in the first year, a similar result to other studies.8,19 he antibody titre immediately after completing vaccination is the major predictive factor of immunity maintenance in these patients.19,22 Thus, 75% of the patients with antibodies > 1,000UI/ml maintained seroprotection four years after vaccination, while this percentage was less in patients with lower antibody titres. A striking fact in our study was not finding significant differences in the maintenance of immunological memory among patients with initial titres between 11-99 and 100-999UI/ml. An antibody titre of 100UI/ml is considered as the necessary threshold to maintain adequate immunological protection in immunocompromised patients.13 In our study, in the long-term, this titre did not offer greater seroprotection than inferior titres, which suggests that other factors, independent of the initial vaccination response, could influence in the maintenance or loss of immunological memory.
Current recommendations in the follow-up of vaccinated patients against HB suggest carrying out annual anti-HB antibody tests,16 although there is no unanimity in relation to the frequency of these tests. Indeed, there is great variability in the frequency of these tests in different dialysis services.23 Furthermore, due to the elevated percentage of patients who lose their immunological memory and that the loss of antibodies can be premature, it has been suggested that it may be useful in these patients to perform more frequent tests, 6-12 months after vaccination.24 In our study, annual antibody testing makes the elapsed time between the last vaccine dose and the determination of the antibody rate very variable and those patients classified as non-responding, in reality, could be responding patients with a low rate of antibodies and fast disappearance of this rate. Indeed, our results show that biannual testing could facilitate detection of a large number of patients who could benefit from a biannual booster dose instead of an annual one and, moreover, would expedite correct identification of the vaccination response in these non-responding patients.
To sum up, in our experience, the administration of three double doses of recombinant vaccine against HB immunises half of the HD patients. Age is a determining factor in the initial response to vaccination. One third of the patients lose their immunological memory in the first year of follow-up. The antibody titre after completing the vaccination protocol is a predictor of the maintenance of this memory. The administration of booster doses immunises one fourth of the non-responding patients and biannual testing can be of use to better control and manage vaccinations in this population.
Table 1. Initial response to the hepatitis B vaccine
Table 2. Additional booster doses in non-responding patients
Figure 1.