To the Editor:
Teriparatide is a fragment of the natural human parathyroid hormone (PTH) consisting of the first 34 amino acids counting from the N-terminus end of the natural PTH. It is obtained by bioengineering in Escherichia coli. The peptide interacts with the PTH type 1 receptor, which is mainly located on osteoblasts and in renal tubules cells. Intermittent therapy with teriparatide increases the number of osteoblasts and subsequent bone formation, an effect that is mediated by the decrease in osteoblast apoptosis and an increased activation of osteoblasts and preosteoblasts, which is why it is used in the treatment of osteoporosis with a high risk of fracture.1-2 Thus, it could also be observed that in renal transplant patients with hypoparathyroidism, where hypocalcaemia may worsen with the use of steroids, using synthetic PTH is a therapeutic option because it improves bone mineralisation, decreasing its resorption and the excretion of urinary calcium and increasing its intestinal absorption.3-4
Our aim is to assess subcutaneous teriparatide treatment in renal transplant patients with symptomatic post-operative hypoparathyroidism.
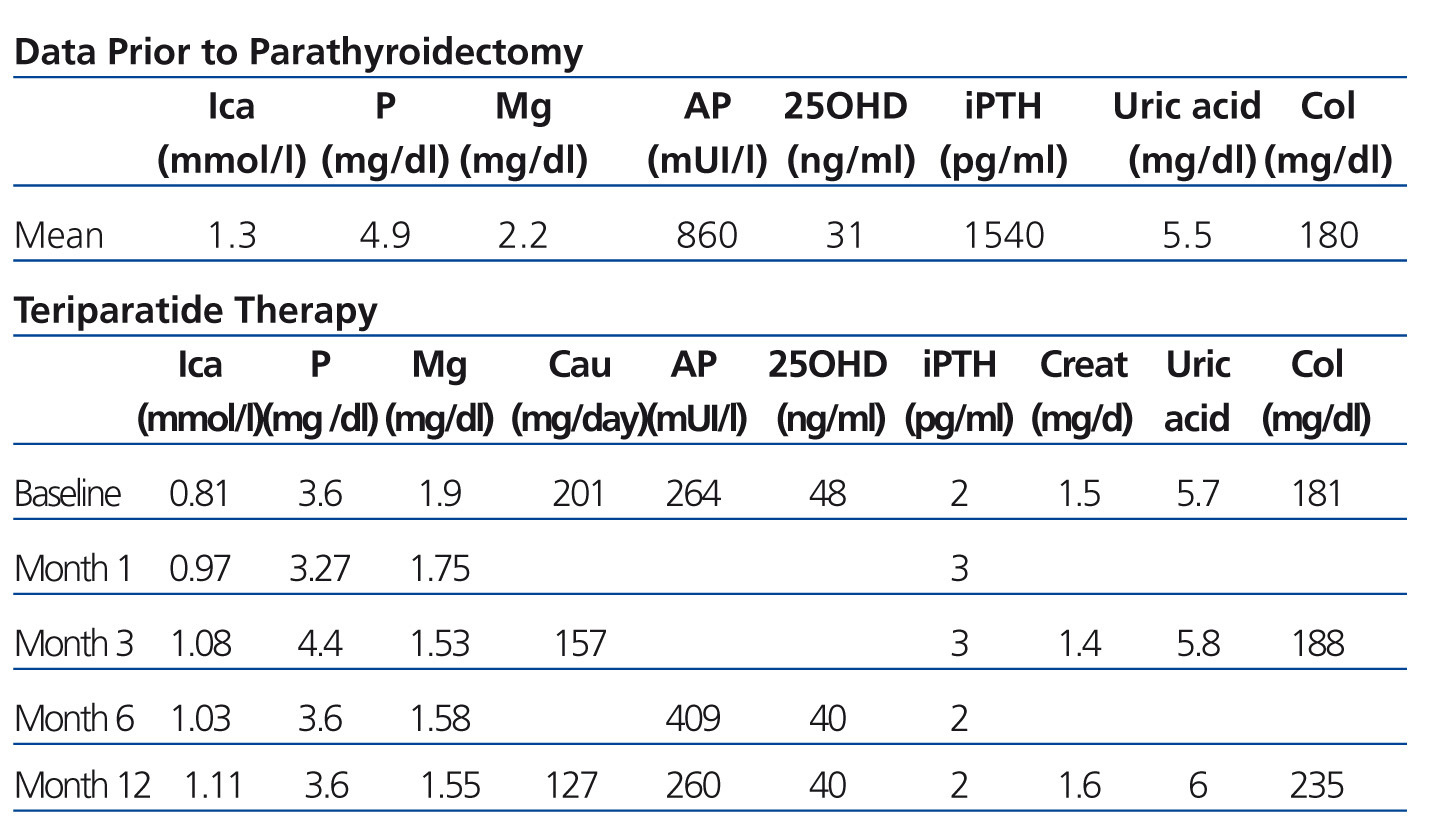
We report subcutaneous teriparatide treatment in doses of 20ug/day in three deceased-donor renal transplant patients with symptomatic post-operative hypoparathyroidism from January 2012. The following biochemical parameters were determined (at baseline, at 1, 3, 6 and 12 months post-treatment): ionic calcium (normal values [NV] 1-1.30mmol/l), phosphataemia (NV 2.5-4.50mg/dl), magnesaemia (NV 1.70-2.40mg/day), alkaline phosphatase (NV up to 120-270mIU/l), 24-hour urinary calcium (NV 60-200mg/day), uric acid (NV 3.3-7mg/dl and 2.2-5.7mg/dl in women), cholesterol (NV 150-200mg/dl), iPTH (intact PTH) (NV 35-72pg/ml), 25-hydroxyvitamin D (25OHD) (NV>40ng/ml) and creatinine (0.8-1.2mg/dl).
After transplantation, all patients received immediate induction with thymoglobulin, while subsequent maintenance immunosuppression varied: 2 with calcineurin inhibitors and 1 with sirolimus associated with mycophenolate and deltasone. Patients also received ergocalciferol (according to levels), calcium and calcitriol in high doses.
RESULTS
Three patients with renal transplant from a cadaveric donor (one patient, second transplant) 2 males, (mean) age 42.6±6.02 years, aetiology of chronic renal failure: obstructive uropathy, unknown, systemic erythematosus lupus. (Mean) time on haemodialysis 101.3±121.7 months, time to transplantation 6±3.46 years, surgical parathyroidectomy (PTX) one patient prior to transplantation, two patients in the fourth year after transplantation, all due to severe secondary hyperparathyroidism. In the PTX immediate postoperative period, two patients had seizures due to hypocalcaemia, in one there was a fall from standing height and fracture of the scapula and clavicle. Patients persisted in their progression with hypoparathyroidism (PTH <5ng/ml) with symptomatic hypocalcaemia that was difficult to correct. Requirement for high doses of calcium and calcitriol orally and intravenously and ergocalciferol orally (maintaining 25OHD values at 40ng/ml).
Treatment began with synthetic PTH in January 2012 due to symptomatic hypoparathyroidism and difficulty in taking the medication (calcium and calcitriol in high doses).
Once teriparatide was administered to patients, the requirement for calcium and calcitriol was minimal, and they were subsequently discontinued (30 days).
The biochemical parameters are shown in Table 1.
As regards adverse events: at the beginning of the treatment, only one patient had nausea, mild hypotension and numbness in legs, the latter being due to hypomagnesaemia.
One patient, at the ninth month of treatment, reported an episode of shivering and tachycardia, in addition to dyspnoea during the administration of the following day. The patient sought consultation due to this event and it was decided to discontinue the medication. He did not have symptoms in the following days.
According to what has been described in literature, antibodies have been detected that had crossed reactivity with teriparatide in 2.8% of the women treated with the aforementioned drug, generally after 12 months of treatment, with symptoms clearing after the discontinuation of the latter.
We did not observe abnormalities in the values of 24-hour urine calcium and creatinine, but there was a slight increase in uric acid, cholesterol and alkaline phosphatase (PTH effect).
CONCLUSION
Synthetic PTH in intermittent treatment is a therapeutic option for patients with hypoparathyroidism. It improves symptoms, corrects calcaemia values and reduces requirements for calcium and calcitriol, and in short, helps to improve the quality of life that we must offer our transplant patients.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Biochemical data prior to parathyroidectomy and during teriparatide therapy