La glomerulonefritis (GN) membranoproliferativa puede asociarse patogénicamente con la infección por el virus de la hepatitis C (VHC), como numerosos casos clínicos han mostrado. La posible relación entre VHC y GN IgA, por el contrario, ha sido sugerida solamente en casos aislados. La nefropatía IgA recurre hasta en un 50 % de los casos tras el trasplante renal, pero es poco frecuente que aparezca como una GN de novo. Presentamos el caso de un paciente con infección crónica por VHC y hepatopatía, que desarrolla dos patologías glomerulares diferentes a lo largo de su evolución: GN membranoproliferativa en sus riñones nativos, proceso que causó una insuficiencia renal terminal, y GN IgA de novo en el riñón trasplantado. Se discute la posible relación patogénica de ambos procesos glomerulares con la infección por VHC.
Membranoproliferative glomerulonephritis (GN) may be pathogenically associated with infection due to the hepatitis C virus (HCV) as many clinical cases have shown. The potential relationship between the HCV and IgA GN, by contrast, has been suggested only in isolated cases. IgA nephropathy recurs in up to 50% of cases after renal transplantation, but it is uncommon for it to appear as a de novo GN. We report the case of a patient with chronic infection due to the HCV and liver disease, who developed two different glomerular diseases during its progression: membranoproliferative GN in his native kidneys, a process that caused terminal renal failure and de novo IgA GN in the transplanted kidney. The potential pathogenic relationship of both glomerular processes with infection due to the HCV is discussed.
INTRODUCTION
De novo glomerulonephritis (GN) is defined as a glomerular disease that appears after a renal transplantation has been performed and which is different from the primary renal disease. The most common de novo GN are membranous nephropathy, focal segmental glomerulosclerosis, membranoproliferative GN (MPGN) and thrombotic microangiopathy secondary to drug intake. Other forms, such as de novo IgA GN are uncommon.1,2
In recent decades, cases of GN associated with the presence of the hepatitis C virus (HCV), mainly MPGN and membranous GN, have been described.2-6 The relationship between IgA GN and the aforementioned virus has recently been described.7-12
Below, we present the case of a patient who was HCV positive and who developed two different glomerular processes, one in his native kidneys and one in his renal transplant, both related to the aforementioned virus.2,7-13
DISCUSSION OF THE CLINICAL CASE
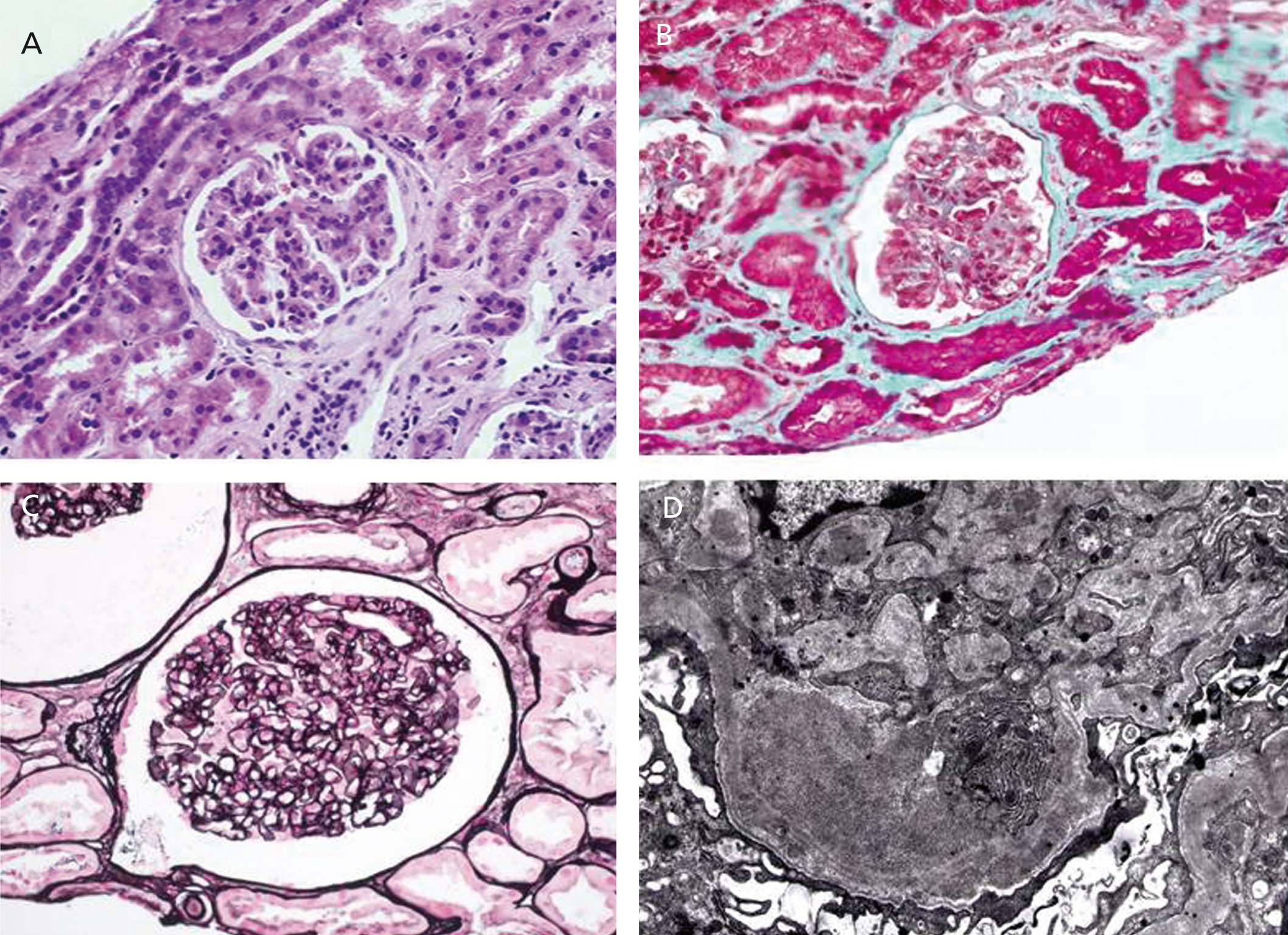
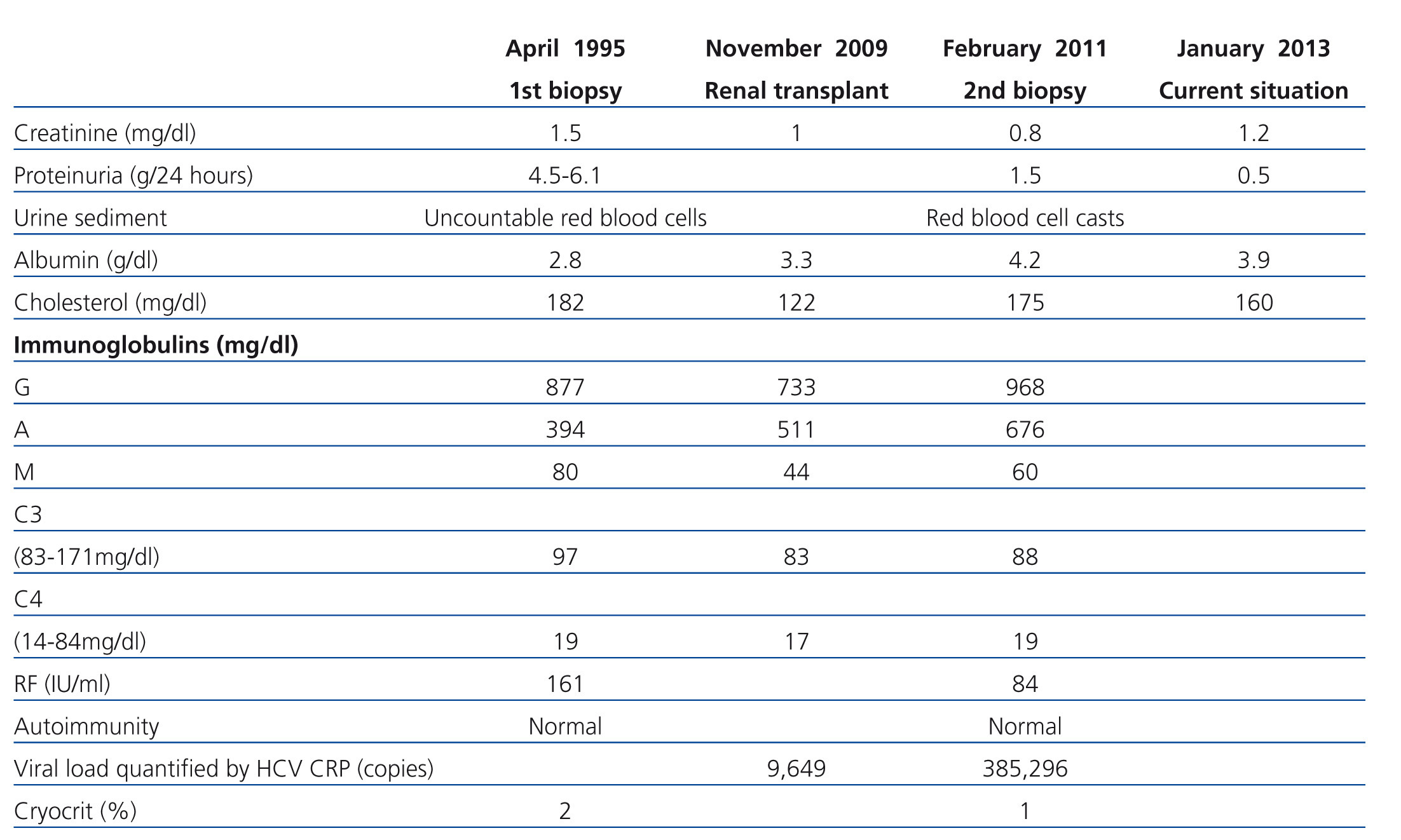
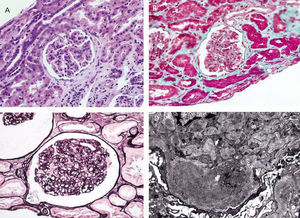
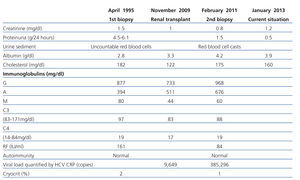
A 56 year-old male with a personal history of alcoholic hepatitis, who in 1995 was admitted for a nephrotic syndrome, microhaematuria and renal failure (creatinine 1.5mg/dl) study. The physical examination revealed the presence of oedemas, hepatomegaly and purpuric lesions on his lower limbs. The study (Table 1) showed a positive serology for the HCV, with the remaining serologies being negative. It also revealed positive cryoglobulin. A renal biopsy was performed, revealing a renal parenchyma with seven glomeruli with diffuse involvement, increased cellularity and a mesangial matrix with a lobular appearance, basement membranes with images of double contour, irregular thickening of the capillary walls and interstitial fibrosis. Direct immunofluorescence revealed C3 granular parietal glomerular deposits and the electron microscopy showed subendothelial deposits, swollen endothelial cells and mesangial expansion (Figure 1). The patient was diagnosed with MPGN associated with HCV with positive cryoglobulins.
The patient was referred to the Digestive System Clinic to assess HCV treatment on a whole. A liver biopsy was performed, which revealed cirrhosis of the liver (P-3, L-2, F-3). Given these results, we started treatment with interferon alpha, initially without achieving a sustained viral response, and subsequently with its pegylated form for 48 weeks achieving a negative viral load. Four months after completion of treatment, he had an episode of acute pericarditis with presence of HCV in the pericardial fluid coinciding with a further increase in viral load. The patient had had poor clinical tolerance to both previous treatments and it was decided not to initiate further treatment. Another complication that he presented was inflammatory arthritis of his major joints.
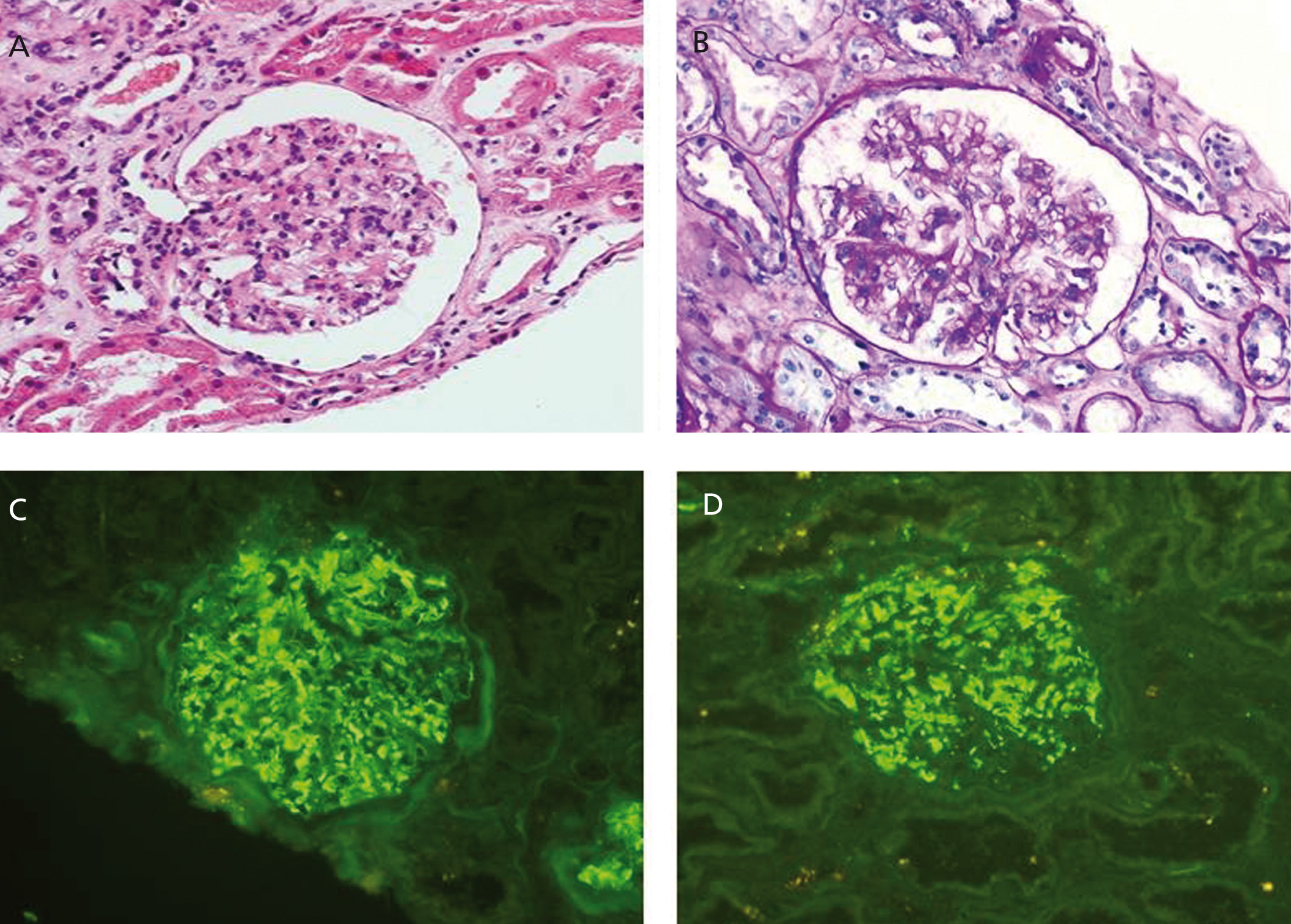
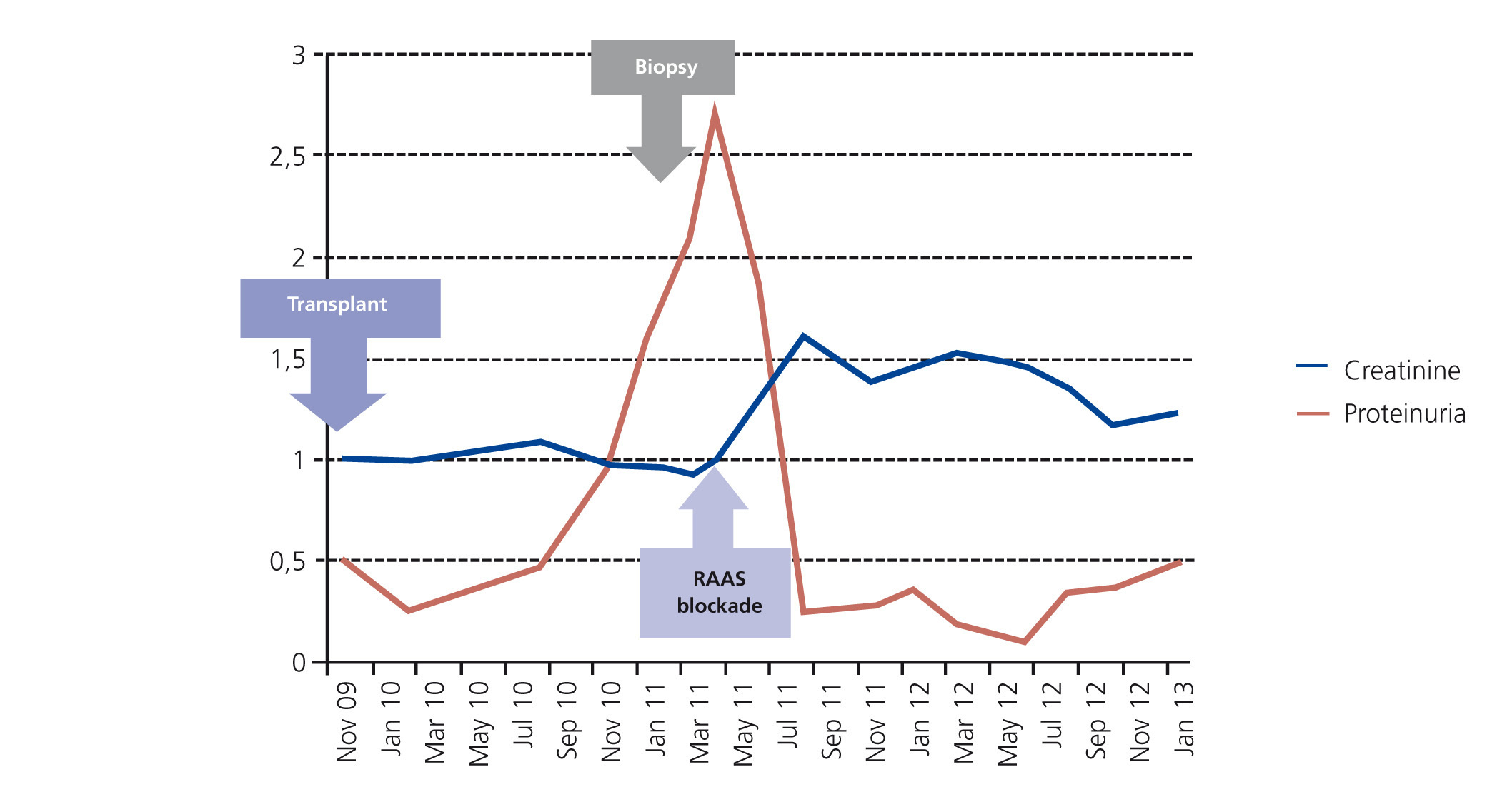
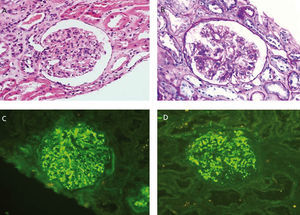
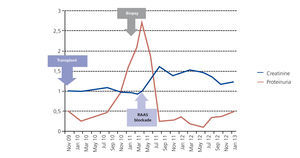
From a renal perspective, antiproteinuric therapy was introduced with renin-angiotensin-aldosterone system blockade, despite which the patient developed progressive renal failure and finally began haemodialysis in 2007. In November 2009, he received his first kidney transplant from a donor who had died from spontaneous cerebral haemorrhage. The donor had HLA typing: DR1, DRX, B14, B35, A11, A30, and a history of alcoholism, with creatinine in the moment of removal of 1mg/dl and negative proteinuria. Immunosuppression was performed with corticosteroids, mycophenolic acid and tacrolimus. Renal progression was excellent and there was immediate renal function. Thirteen months after transplantation, the patient developed non-nephrotic proteinuria and microhaematuria with red blood cell casts, while maintaining normal renal function. An autoimmunity study was performed (Table 1); donor-specific antibodies, cytomegalovirus antigenaemia and serum CRP (polymerase enzyme chain reaction) levels for the BK virus were negative, and the renal Doppler ultrasound was normal. Given the suspected recurrence of the underlying disease, we opted for a renal biopsy. The optical microscopy showed a renal parenchyma with ten glomeruli with marked mesangial expansion, mild fibrosis and tubular atrophy. Immunofluorescence revealed granular mesangial IgA and C3 deposits, and electron microscopy showed mesangial expansion of cells and matrix with abundant homogeneous electron deposits. The C4d immunohistochemistry techniques were negative (Figure 2). The patient was diagnosed with de novo IgA mesangial GN in the graft, and the dose of corticosteroids and mycophenolic acid were increased without clinical biochemical improvement. After the start of antiproteinuric treatment with blockade of the renin-angiotensin-aldosterone system by angiotensin receptor antagonist (ARA II) and the subsequent association of spironolactone, a negative proteinuria result was achieved, although with a small deterioration renal function that is currently stabilised (Figure 3).
DISCUSSION
De novo GN in the renal graft are defined as a glomerular disease that appears after a renal transplantation, which is different from the primary nephropathy and they are the third most common cause for loss of graft at 10 years after transplantation. Their prevalence in the publications is highly variable due to: the different criteria and protocol for carrying out renal biopsies, the fact that in some cases there is only optic microscopy without immunofluorescence or electron microscope, not being able to carry out the differential diagnosis safely with other forms of the disease, the total count of de novo GN and recurrences of the original disease and the presence of a renal disease of unknown origin. The most common forms are membranous nephropathy, focal segmental glomerulosclerosis, MPGN, thrombotic microangiopathy secondary to drug intake and nephritis due to basement membrane antibodies, associated with Alport’s syndrome.1,2 The presence of de novo IgA GN is uncommon.14-19 With regard to the clinical course, de novo GN appear late, normally from one year after the transplantation, in contrast to recurrences, which usually appear in the first weeks after transplantation.1 Graft survival, both in recurrences and in de novo GN is clearly lower than in patients who do not develop any glomerular process.20
We report the case of a patient with nephrotic syndrome, in whose study we note a positive serology for the HCV and positive cryoglobulins. The renal biopsy shows an MPGN associated with a chronic infection due to the HCV. This entity may appear in a primary form (idiopathic) or as secondary to the deposit or in situ formation of immune complexes in the glomerulus, caused by diseases with chronic activation of the immune system (such as infections due to the hepatitis B and C viruses), autoimmune diseases, and the inability to remove immune complexes and some tumours.21
After receiving his first renal transplant, the patient displayed proteinuria and microhaematuria with red blood cell casts, and a recurrence of his baseline disease was initially suspected, which occurs in 20-30% of cases,1,2 but the histopathological study revealed the presence of a de novo IgA GN. The aetiology of the second GN is uncertain. Initially, it could be related to the presence of chronic liver disease in the recipient.13 Secondly, the possibility is considered that the donor has a non-diagnosed IgA GN without proteinuria at the time of transplantation. The donor had a history of alcoholism and was a carrier of HLA-B35, which are factors associated with this glomerulopathy,13,22 although, we do not know if they had previously presented abnormalities in renal function or urinary sediment. No renal biopsy was carried out on the graft before the transplantation and the contralateral kidney was not transplanted because it carried a renal cyst. This possibility seems more remote to us, given that what is described in literature suggests that IgA deposits in the transplanted kidney disappear soon after transplantation.23
The HCV is a small RNA virus (30-38nm) with a lipid envelope and it belongs to the Flaviviridae family. This virus may result in the appearance of some renal diseases. Furthermore, renal disease patients have a higher risk of becoming infected by the HCV because they have greater direct or indirect contact with blood from other patients (transfusions, haemodialysis, transplantations). In haemodialysis centres in the United States, a prevalence of 8-10% of patients infected by HCV and an incidence of 1-3% new cases per year has been described. It has been related to different renal diseases, however, the most common are type I MPGN, which is associated with type II mixed cryoglobulinaemia and to a lesser extent, non-cryoglobulinaemic MPGN and membranous GN. Small series and isolated cases have also been described that associate infection due to HCV with other processes, both glomerular (focal and segmental glomerulosclerosis, immunotactoid and fibrillary glomerulopathy, acute post-infectious GN and IgA GN7-12) and non-glomerular (tubulointerstitial nephritis and thrombotic microangiopathy).3,11
This history may suggest that both glomerular processes in our patient are associated with infection due to HCV. In the first GN, MPGN, the association with the virus is well documented and supported by many epidemiological studies, clinical cases, trials and experimental work.3,24 The relationship between the HCV and the second glomerular process, de novo IgA GN, is less common. At the start of the 1990s, the link between the HCV and this GN was not known, although the link between the latter and chronic alcoholic liver disease was known.25,26 However, over the years, isolated cases7-10 and series of cases11 of IgA GN in native kidneys have been described. There are also cases of patients in whom, after completing the antiviral treatment with interferon and ribavirin and achieving a sustained viral response, the liver profile and the urinary sediment normalised.7,27 The prevalence of IgA GN in HCV patients varies between 0% and 6% according to the series, although in other geographical regions such as Saudi Arabia or Japan, there are greater frequencies.10,11,28 Sansonno et al. have confirmed the presence of viral RNA in the glomeruli and viral core particles both in glomeruli and tubules of IgA patients, although in a lower percentage than in other glomerulopathies such as MPGN and membranous GN. These data suggest that the HCV has a role in the pathogenesis of IgA GN in these patients.29
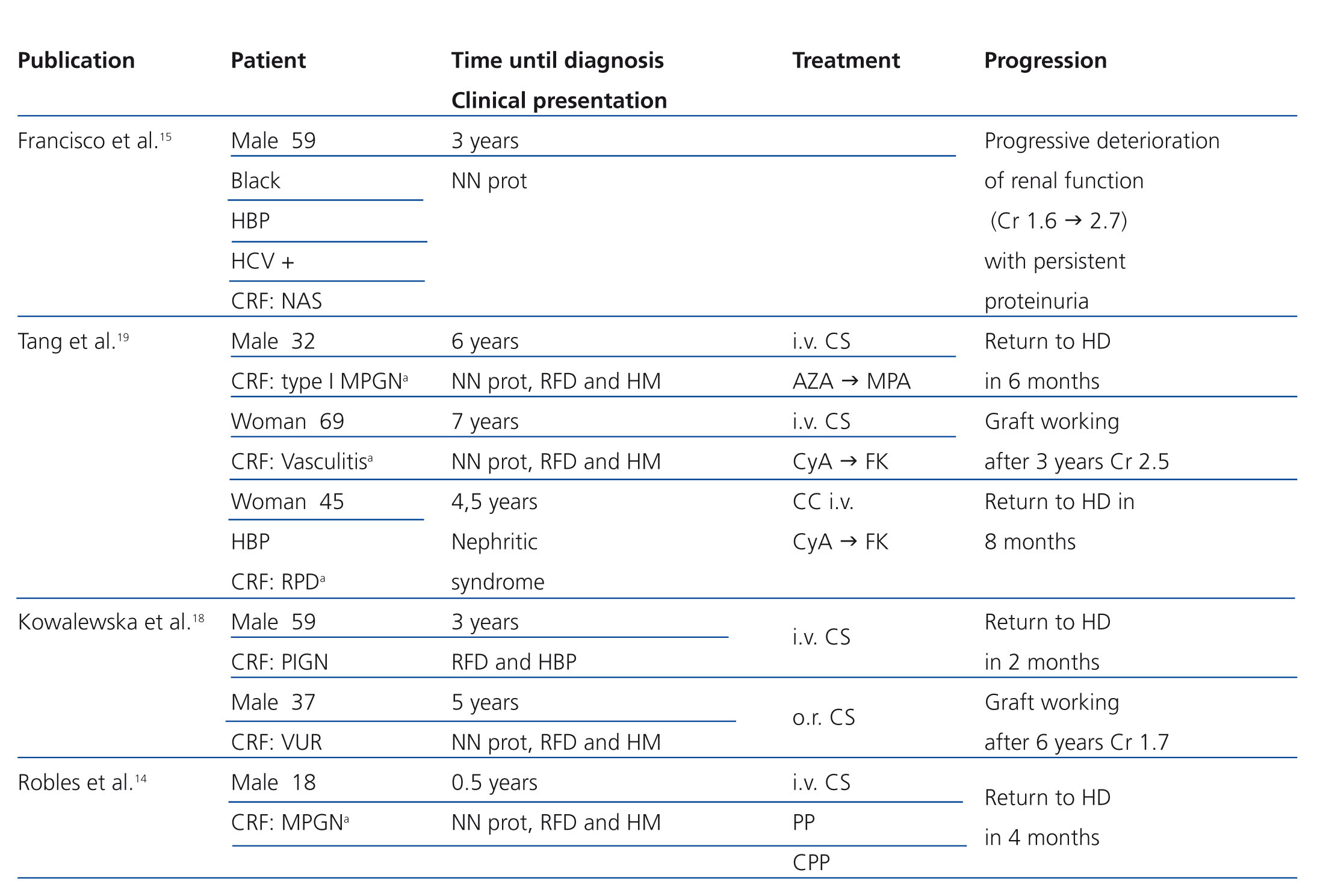
Various renal diseases have been described in patients who have the HCV after receiving a renal transplant (MPGN, membranous GN, minimal change disease, thrombotic microangiopathy, acute rejection, chronic rejection). With regard to de novo GN, the HCV was associated with membranous GN and MPGN.3-5 Its potential aetiological role in de novo IgA GN is unknown. We displayed the cases described in literature of IgA GN, along with their progression in Table 2. There is a notable case described by Francisco et al. of a black chronic kidney disease patient attributed to nephroangiosclerosis secondary to non-biopsied high blood pressure, without haematuria. The patient acquired the infection due to the HCV after receiving various transfusions when he was on renal replacement therapy, in the form of haemodialysis, developing chronic liver disease. He eventually received a renal graft from a deceased donor. A renal biopsy was carried out two weeks after the transplant, and it displayed an acute rejection without lesions or deposits suggestive of IgA GN. After three years, he developed non-nephrotic range proteinuria, and a renal biopsy was carried out with IgA GN being diagnosed.15 In the other cases of de novo IgA GN described, we do not have recipients’ HCV serology data. In our case, the diagnosis of de novo IgA coincides with an increase in the HCV viral load, which indicates active RNA replication. This, along with the effect of immunosuppression and the virus itself could modulate the response of lymphocytes and the production of antibodies, thus generating an imbalance between antigens and antibodies which, along with the susceptibility of the renal graft, would influence the long-term genesis of de novo GN.5
Lastly, we wish to highlight the favourable progression made by our patient, similar to that presented in other cases of de novo IgA GN without crescents in the renal biopsy, a marker of poor prognosis in renal graft relapses.14-19 Our patient did not display crescents but was HCV positive, which is described as a risk factor for the development of proteinuria, chronic rejection, glomerular lesions, infections and decrease of the survival, both of the graft and the receiver.5,6De novo IgA GN appeared while the patient was being treated with the triple immunosuppressant therapy (corticosteroids, mycophenolic acid and tacrolimus). However, the control of proteinuria was not achieved despite the increase in the dose of steroids and mycophenolic acid. Lastly, blockade of the renin-angiotensin-aldosterone system was carried out by ARA II and spironolactone, with a decrease in proteinuria but a slight deterioration in renal function, which is currently stable. The patient is being treated with mycophenolic acid as part of his immunosuppressant therapy, although the use of mycophenolic acid in the treatment of IgA nephropathy is still controversial.30
In summary, in this pathogenesis, the HCV is related to different renal diseases, amongst them MPGN and IgA GN. In our case, we believe that infection due to the HCV could have contributed to the pathogenesis of both glomerular processes, although, we cannot rule out that de novo IgA GN could be secondary to chronic liver disease.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Native kidney biopsy
Figure 2. Biopsy of the renal transplant
Figure 3. Progression of renal function and proteinuria
Table 1. Analytic progression and complementary tests
Table 2. Review of the cases of de novo IgA glomerulonephritis in patients with renal transplants