The systemic bioimpedance vector analysis (BIVA) is a method widely utilized to assess body composition and systemic volume overload in both patients with heart failure and advanced renal failure. Recent studies have shown the superiority of BIVA in the estimation of fluid overload over strict clinical criteria during haemodialysis sessions.1 The use of BIVA in this context helps to improve fluid control, blood pressure, quality life scores, and also predicts clinical outcome such as hospitalizations due to cardiovascular causes.2
The population with cardiac stimulation devices increases every year, particularly within our patient population. The annual consumption of conventional generators and cardiac resynchronization devices with or without defibrillator is estimated to be 745.8 and 53.1 units per million inhabitants respectively. This is the most recent data obtained by the Spanish Society of Cardiology, considering that there are 35,000 implants per year in Spain.3
Although it is not well known whether BIVA can affect the function of cardiac stimulation devices, most manufacturers and even clinical practice guidelines4 advice against its use in this group of patients, due to the risk of electrical interference that may result in signal oversensing, stimulation inhibition, inappropriate defibrillator shocks or other functional anomalies. These restrictions limit the use of BIVA in many patients with a history of cardiopathy, particularly for those with cardiac failure.
As a consequence, we decided to assess if BIVA caused electromagnetic interference or generator malfunction in patients with cardiac stimulation devices. To do this, all admissions to our centre due to acute decompensation of cardiac failure between January and June 2014 were prospectively studied. The analysis was performed prior to discharge, using a CardioEFGTM model at a frequency of 50kHz. The study was authorized by the Clinical Trials and Research Committee of our centre. Out of 77 patients studied, 21 had cardiac device (Table 1). Prior to BIVA, all generators and wires showed normal function. During BIVA, it was not detected any effects on intracavitary electrocardiograms, we did not observe any inappropriate sensing in channels. No telemetric interferences were observed between the cardiac device and the programming device either. Upon repetition of the interrogation after BIVA, no changes in the wire parameters nor alterations in the functioning of the generator device were detected in any of the patients.
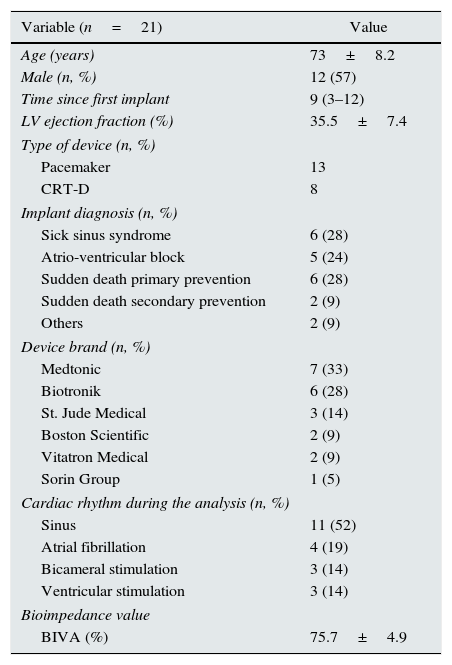
Characteristics of the study population.
| Variable (n=21) | Value |
|---|---|
| Age (years) | 73±8.2 |
| Male (n, %) | 12 (57) |
| Time since first implant | 9 (3–12) |
| LV ejection fraction (%) | 35.5±7.4 |
| Type of device (n, %) | |
| Pacemaker | 13 |
| CRT-D | 8 |
| Implant diagnosis (n, %) | |
| Sick sinus syndrome | 6 (28) |
| Atrio-ventricular block | 5 (24) |
| Sudden death primary prevention | 6 (28) |
| Sudden death secondary prevention | 2 (9) |
| Others | 2 (9) |
| Device brand (n, %) | |
| Medtonic | 7 (33) |
| Biotronik | 6 (28) |
| St. Jude Medical | 3 (14) |
| Boston Scientific | 2 (9) |
| Vitatron Medical | 2 (9) |
| Sorin Group | 1 (5) |
| Cardiac rhythm during the analysis (n, %) | |
| Sinus | 11 (52) |
| Atrial fibrillation | 4 (19) |
| Bicameral stimulation | 3 (14) |
| Ventricular stimulation | 3 (14) |
| Bioimpedance value | |
| BIVA (%) | 75.7±4.9 |
Results expressed as median±standard deviation (quantitative variables), or in numbers and percentages (qualitative variables). CRT-D: cardiac resynchronization therapy with defibrillator; LV: left ventricle; BIVA: systemic bioimpedance vector analysis.
Literature on the safety of bioimpedance analysis of the body in patients with cardiac stimulation devices is scarce, although due to widespread use evidence of safety is needed. Recently, Buch et al.5 found no alterations in the generators during the use of BIVA, in a cohort of 20 outpatients with chronic cardiac failure. In this study, as in ours, there is a limited number of patients and the use of a single system of analysis. Nevertheless both studies suggest that the use of BIVA in patients with cardiac devices is safe within this context.
Please cite this article as: Fabregat-Andrés Ó, Fácila L, Montagud-Balaguer V, Galán-Serrano A. Análisis de bioimpedancia sistémica en pacientes portadores de dispositivos de estimulación cardiaca. Nefrologia. 2015;35:345–346.