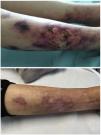
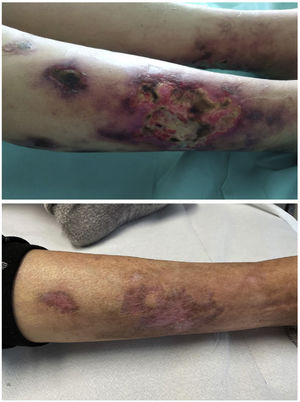
Calciphylaxis, also known as calcific uraemic arteriolopathy (CUA), is a serious and uncommon complication in patients on chronic haemodialysis.1 In its pathogenesis, in addition to the involvement of bone-mineral metabolism, the possible deleterious role that certain medium-sized molecules could play has been hypothesised.2 Expanded haemodialysis (HDx) facilitates the effective removal of this type of molecules, so we believe that they could play a role in the management of this condition.
We present the case of a 47-year-old woman with a history of long-standing and poorly-controlled type 1 diabetes mellitus, arterial hypertension, chronic ischaemic heart disease, and severe aortic stenosis, who required aortic valve replacement with initiation of anticoagulation with acenocoumarol two months before admission. The patient also had secondary hyperparathyroidism and chronic kidney disease category G5 according to KDIGO 2012 of probable diabetic aetiology, receiving chronic haemodialysis through a radiocephalic arteriovenous fistula placed in the left upper limb.
She reported a two-week history characterised by the presence of initially small and erythematous lesions that progressed to being ulcerative, some of them were circular with a blackish centre, very painful, and with exudate that was positive for Pseudomonas aeruginosa. A skin biopsy of one of the lesions was consistent with calciphylaxis, so the patient was admitted to nephrology ward. The following blood test results were obtained: procalcitonin 1.49 ng/m; C-reactive protein 26.6 mg; neutrophil-to-lymphocyte ratio (NLR) 5.75; platelet-to-lymphocyte ratio (PLR) 413.70 and systemic immune-inflammation index (SII) 1,737; calcium 10.30 mg/dl; phosphate 5.05 mg/dl; biointact parathyroid hormone (PTH) (1–84) 490 pg/m and 25-OH-vitamin D 10.8 ng/m. Cervical ultrasound showed a hypoechoic nodule 1.38 cm in diameter suggesting parathyroid gland hyperplasia vs hypertrophy.
Joint management with dermatology was decided upon with treatment every 48 hours with topical sodium thiosulfate, in addition to intravenous sodium thiosulfate at a dose of 12.5 g post-haemodialysis (three times a week). The dialysis dose was intensified with daily 210-minute sessions and changed to expanded haemodialysis with Theranova 500® 2 m2 filter (Baxter International Inc., Deerfield, IL, USA) with a mean Qb of 313 ml/min, a mean Qd of 500 ml/min and a mean Kt of 41 L. Among other measures, the patient was changed to anticoagulation with enoxaparin and her treatment with paricalcitol, vitamin D and iron was suspended. Management of the patient's secondary hyperparathyroidism was optimised with cinacalcet, non-calcium-based phosphate binders and a low-calcium dialysis bath (1.25 mEq/L). Combination antibiotic therapy with ceftazidime and vancomycin was administered.
At discharge, there was evidence of improved inflammatory parameters along with favourable skin lesion progression until their complete resolution five months after the start of treatment (Table 1 and Fig. 1). However, we observed a worsening of PTH despite progressively increasing the calcimimetic dose. It was decided to start intradialysis etelcalcetide, pending progression at the present time.
Evolution of the patient's inflammatory parameters and bone-mineral metabolism.
| 2 days before admission | Day 1 | Day 2 | Day 5 | Day 10 | Day 25 | Day 27 | Day 62 | Day 64 | Day 97 | Day 125 | Day 153 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-reactive protein (mg/L) | 31.26 | 35.58 | 26.6 | 25.57 | 8.46 | 3.21 | 0.98 | 1.03 | ||||
| Procalcitonin (ng/mL) | 0.83 | 1.35 | 1.49 | 1.04 | 0.74 | 0.99 | ||||||
| NLR | 7.85 | 5.75 | 6.55 | 5.73 | 10.44 | 2.67 | 3.69 | 8.63 | 3.61 | 3.25 | 3.45 | 3.53 |
| PLR | 564.58 | 413.70 | 378.95 | 362.86 | 425.42 | 237.84 | 205.69 | 261.45 | 253.52 | 248.24 | 215.31 | 205.49 |
| SII | 2,128.48 | 1,737.53 | 1,887.16 | 1,455.06 | 2,620.61 | 704.00 | 933.84 | 1,871.95 | 649.01 | 685.13 | 727.73 | 659.64 |
| Ferritin (ng/mL) | 458.00 | |||||||||||
| Total protein (g/dL) | 6.66 | 6.68 | 6.95 | 7.57 | 6.87 | 6.94 | 6.67 | |||||
| Leukocytes (103/μl) | 5.00 | 5.93 | 6.59 | 5.74 | 7.81 | 5.05 | 6.76 | 8.97 | 3.90 | 4.06 | 4.95 | 4.80 |
| Neutrophils (103/μl) | 3.77 | 4.20 | 4.98 | 4.01 | 6.16 | 2.96 | 4.54 | 7.16 | 2.56 | 2.76 | 3.38 | 3.21 |
| Lymphocytes (103/μl) | 0.48 | 0.73 | 0.76 | 0.70 | 0.59 | 1.11 | 1.23 | 0.83 | 0.71 | 0.85 | 0.98 | 0.91 |
| Haemoglobin (g/dL) | 7.80 | 8.50 | 10.70 | 10.00 | 10.80 | 10.90 | 11.20 | 10.90 | 11.00 | 10.10 | 10.20 | 10.80 |
| Platelets (103/μl) | 271.00 | 302.00 | 288.00 | 254.00 | 251.00 | 264.00 | 253.00 | 217.00 | 180.00 | 211.00 | 211.00 | 187.00 |
| Calcium (mg/dl) | 10.19 | 10.30 | 9.64 | 9.77 | 9.79 | 10.18 | 10.06 | 9.92 | ||||
| Phosphates (mg/dl) | 6.78 | 5.05 | 2.72 | 4.90 | 4.45 | |||||||
| Biointact PTH (1-84) (pg/mL) | 490.00 | 315.00 | 824.00 | 1,046.00 |
NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio: SII: systemic immune-inflammation index.
Despite correct treatment, a large percentage of patients die (35% in one year, despite treatment and 55% if not treated).3 It has been reported that the alteration of bone-mineral metabolism is the main predisposing factor for this disease.1 However, this case highlights the possible role other non-traditional factors might play given the persistence of hyperparathyroidism over time, despite there being substantial and sustained improvement in both lesions and inflammatory parameters.
Intravenous and topical thiosulfate could play a very important role in this regard. However, this may not be enough to explain the patient's rapid inflammatory and cutaneous improvement. In this sense, it should be noted that within the pathophysiology of the disease, the involvement of both an excess of cytokines and coagulation alteration has been postulated.4 The possible role of adipokines deserves special mention, including Vascular Endothelial Growth Factor A (VEGF-A), which induces calcification and could explain the higher frequency of calciphylaxis in POEMS syndrome.5 Similarly, leptin, another adipokine, has been linked to osteoblast promotion and smooth muscle cell mineralisation.2 These two proteins weigh 46 kDa and 16 kDa, respectively, and this places them within the group of medium-sized molecules6, which are the main target of expanded haemodialysis through the new medium cut-off membranes.
Therefore, in the absence of larger studies, we conclude that expanded haemodialysis could be a good complement to thiosulfate treatment in the management of calciphylaxis in patients on chronic haemodialysis.
Please cite this article as: Valga F, Monzón T, Rincón M, Vega-Díaz N, de la Flor JC, Aladro-Escribano S et al. ¿Sinergia del tratamiento con tiosulfato sódico y hemodiálisis extendida en el manejo de la calcifilaxis? A propósito de un caso. Nefrologia. 2022;42:354–356.