Acute poisoning in children is a frequent cause of admission to emergency units and can lead to death and acute kidney injury (AKI). The incidence of poisoning in children varies from 0.33% to 7.6%.1 Naphthalene poisoning can lead to a severe clinical Picture.2 AKI due to naphthalene poisoning is very uncommonly reported in the literature, especially if it requires renal replacement therapy. Acute intravascular hemolysis may be the major mechanism of AKI in naphthalene poisoning.3,4 In this study, we described a rare case of AKI following naphthalene poisoning in a pediatric patient successfully treated.
A 1-year-old girl was admitted to the emergency department after accidental consumption of naphthalene balls. The time elapsed until emergency admission was five hours from the ingestion. The patient presented with severe abdominal pain, emesis, serious general condition, and a decreased consciousness level.
In the emergency unit, gastric lavage and activated charcoal were administered. Moreover, she received primary medical care and vigorous intravenous hydration. Results of initial laboratory tests were in the normal range (Table 1), and initial estimated glomerular filtration rate (eGFR) was 51mL/min/1.73m2. Nine hours later, the patient had acute respiratory failure, requiring intubation and ventilatory support. The patient had a left hemorrhagic pleural effusion and severe and acute anemia. Transfusion therapy (1 unit of packed red blood cells) was required and a thoracic surgeon performed chest drainage due to hemothorax.
Laboratory findings on admission and during the follow-up of the patient.
| Parameters | Admission | 9h | 24h | 3rd Day | 5th Day | 10th Day | 12th Day | 13th Day | Discharge |
|---|---|---|---|---|---|---|---|---|---|
| Potassium | 4.09 | 4.04 | 4.53 | 4.23 | 3.33 | 5.39 | 6.93 | 5.63 | 3.83 |
| Sodium | 135 | 136 | 130 | 137 | 139 | 137 | 135 | 136 | 134 |
| Urea | 45 | 62 | 55 | 80 | 51 | 67 | 79 | 65 | 69 |
| Creatinine | 0.7 | 1.4 | 1.4 | 2.1 | 1.9 | 2.2 | 2.6 | 1.7 | 1.2 |
| eGFR | 51 | 25.5 | 0 | 0 | 0 | 16.2 | 21 | 32.4 | 30 |
| AST | 75 | 1281 | 738 | 64 | ** | ** | ** | ** | 23 |
| ALT | 22 | 687 | 569 | 139 | ** | ** | ** | ** | 10 |
| DB | 0.23 | ** | 2.87 | 1.31 | 0.33 | ** | ** | ** | ** |
| IB | 0.34 | ** | 3.14 | 1.23 | 1.02 | ** | ** | ** | ** |
| PT | 12.1 | 12.2 | 13.8 | 11.6 | 11.2 | 12 | ** | 12.5 | ** |
| aPTT | 26.7 | 25.9 | 32 | 27.5 | 23.8 | 31.3 | ** | 31.7 | ** |
| D-LDH | ** | 3205 | ** | 972 | ** | ** | ** | ** | ** |
| Hemoglobin | 13 | 11.1 | 10.1 | 8.5 | 7.8 | 7.4 | 8.1 | 9.5 | 10.1 |
| Leukocytes | 12,420 | 7940 | 8020 | 15,820 | 11,170 | 10,230 | ** | 11,000 | 7530 |
| Platelets | 479,000 | 281,000 | 209,000 | 188,000 | 139,000 | 323,000 | ** | 403,000 | 367,000 |
| PH*** | ** | 7.38 | ** | ** | ** | ** | ** | ** | ** |
| pCO2*** | ** | 30.5 | ** | ** | ** | ** | ** | ** | ** |
| HCO3*** | ** | 19.5 | ** | ** | ** | ** | ** | ** | ** |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; D-LDH: D-lactate dehydrogenase; CK: Creatine phosphokinase; PT: Prothrombin time; aPTT: partial time of thromboplastin; DB: direct bilirubin; IB: Indirect bilirubin; eGFR: estimated Glomerular Filtration Rate. ** Not available. ***Arterial Gasometry.
Reference values: Potassium (3.5–5.5mmol/L); Sodium (135–145mmol/L); Chloride (96–109mmol/L); Glucose (74–106mg/dL); Urea (13–43mmol/L); Creatinine (0.6–1.1mmol/L); AST (<32mg/dL); ALT (< 31mg/dL); DB (< 1 UI/L); DI (< 1 UI/L); PT (10–14s); aPTT (22–28s); D-LDH (230–460 UI/L); Albumin (>3.5 UI/L); Hemoglobin 11.3/15.2g/dL); leukocytes (3600–10,000/mm3); platelets (150,000–450,000/mm3); PH (7.35–7.45); pCO2 (35/45mmHg); pO2 (85–100mmHg); HCO3 (22/26mmol/L); BE (−4/+4).
Schistocytes on peripheral smear were demonstrated. Moreover, the increase in indirect bilirubin and D-LDH (D-lactate dehydrogenase) suggested an intravascular hemolysis in progress (Table 1). G6PD levels remained in the normal range. She developed laboratory worsening with abnormal renal markers, suggesting oliguric AKI in progress according KDIGO's criteria (eGFR=14mL/min/1.73m2). The increase in markers in the liver test indicated acute liver damage.
Moreover, she developed signs of hypervolemia and the nephrologist's assistance was requested, who prescribed hemodialysis. Intravenous sodium bicarbonate was used to prevent renal tubular damage due to hemolysis. The patient had hypertension and medications were started. On the 5th day, she began to recover and was successfully extubated. The patient's urine output improved after eleven hemodialysis sessions, and after 20 days, she was discharged as clinically stable. However, she continued with high blood pressure and only partial recovery of her kidney functions was observed (eGFR=30mL/min/1.73m2).
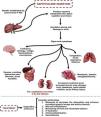
Naphthalene poisoning is a relevant health issue due to the easy availability of this toxic agent, especially in low- and middle-income countries. Small doses of naphthalene in humans can affect various body systems – Fig. 1.5 The patient reported here was a female child presenting with compromised serious general condition, emesis, and abdominal pain due to naphthalene poisoning within a period of 5h from sucking a naphthalene ball. In this patient, the diagnosis of severe acute hepatitis was established from the clinical status, and the liver damage (observed in laboratory tests) should be attributed to naphthalene poisoning.6
Physiopathology and systemic effects following human naphthalene exposure.
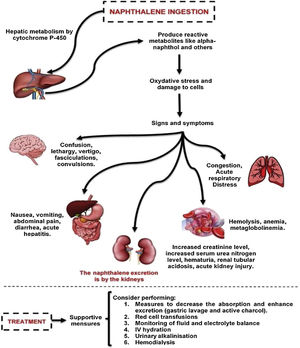
Naphthalene is metabolized in the liver and is oxidized to alpha-naphthol and other metabolites. The cytochrome P450 enzymes are capable of catalyzing these metabolic transformation reactions. Toxic manifestations are mainly due to production of oxygen free radicals leading to lipid peroxidation and deoxyribonucleic acid (DNA) damage, as well as produce oxidative stress. This poisoning could affect various body systems, such as the liver, skin, lungs, kidney, central and peripheral nervous system. The treatment in these cases are mainly supportive.
Hemolysis is very often a consequence of naphthalene poisoning, and it can be worsened by G6PD deficiency. In this case, the diagnosis was suggested by schistocytes on peripheral smear, a high D-LDH level, and increased indirect bilirubin. In addition, G6PD deficiency was absent.
Naphthalene excretion occurs through the kidneys.5 The major pathophysiologic mechanism of AKI is related to intravascular hemolysis and hemoglobinuria.4 Despite the mild hemolytic anemia, the patient had severe AKI and required renal replacement therapy. This fact suggests other mechanisms for AKI development, such as direct nephrotoxicity and acute ischemic tubular necrosis.
There is no specific antidote for naphthalene toxicity and management guidelines are unclear.7 Management is symptomatic with monitoring of fluid and electrolyte balance. Urinary alkalinization has been recommended in the case of hemoglobinuria to prevent it from being deposited in renal tubules.4 The treatment in this case was mainly supportive with mechanical ventilation and blood pressure support with the use of inotropes. Nonetheless, gastric lavage and active charcoal were used to decrease absorption due to the severity of the case.
Hemodialysis is established as an important tool in some life-threatening poisoning cases.8 Hemodialysis was utilized in this case due to the worsening of renal function, oliguria, hyperkalemia, metabolic acidosis, and hypervolemia. Hemodialysis added to the clinical measures promoted the clinical improvement.
Prognosis of naphthalene poisoning is multifactorial.9 In the case reported, the patient had a remarkable improvement and was discharged on the 20th day. However, she continued to show elevated blood pressure.
Accurate examination of the AKI and CKD relationship has important clinical and public health implications. Complex and imperfect processes could follow AKI, subsequently limiting the repair of damaged cells and the return of their normal functioning. This mechanism appears to be one of the responsible factors for the elevated blood pressure and partial recovery of her kidney functions after AKI.10
Acute ingestion of naphthalene is an uncommon cause of poisoning in pediatric patients. Clinicians should be aware of the potential complications in that scenario, such as AKI, and the necessity of early identification, specific management, and follow up of these patients.
Authors’ contributionsKNM, BLC and SMBM contributed to the case data collection. JHHGLP contributed to the draft of the article and the creation of tables and figure. GBSJ, EFD, and PLMMA critically revised the article and approved the version to be published. All authors have read and approved the manuscript.