Antecedentes: regímenes de administración menos frecuentes durante el tratamiento de la anemia podrían beneficiar a los pacientes en Diálisis Peritoneal (DP). Se ha investigado la eficacia de darbepoetin alfa administrado quincenalmente (C2S) para mantener los niveles de Hemoglobina (Hb) (11-13 g/dl). Pacientes y métodos: en este estudio observacional, prospectivo y de ocho meses de duración, participaron 109 pacientes en DP procedentes de 14 centros. Los pacientes (Hb 11-13 g/dl) tratados semanalmente (CS) con darbepoetin alfa pasaron a un régimen de dosificación quincenal (C2S) a criterio del investigador. Las dosis se ajustaron según las directrices vigentes. Resultados: un 69% (75 de 109) de los pacientes cambió a la dosificación C2S. Un 33% mantuvo la dosis semanal media previa 26,1-25,8 μg/semana (equivalente al doble de la dosis CS previa). Un 47%, recibió una dosis reducida (35,8-20,2 equivalente a la dosis CS previa). Un número mayor de pacientes en el grupo de mantenimiento 11 g/dl en comparación con los pacientes de la dosis presentó niveles de Hb tratados con una dosis semanal reducida (80 frente a 51,4%, respectivamente; p = 0,0236). Durante la fase C2S, el nivel medio de Hb osciló entre 12,0 y 12,5 g/dl en el grupo con mantenimiento de la dosis y entre 11,5 y 12,0 g/dl para el grupo con reducción de dosis. Desde la conversión hasta el final del estudio, el cambio medio (DE) en los niveles de Hb fue de -0,7 g/dl (0,98 g/dl, p = 0,0557) y -0,6 g/dl (1,6 g/dl, p = 0,1296) para los grupos con mantenimiento y reducción de dosis, respectivamente. Darbepoetin alfa administrada quincenalmente fue bien tolerada. Únicamente tuvo lugar un solo acontecimiento adverso relacionado con el tratamiento (policitemia). Conclusión: la mayoría de pacientes en DP tratados con darbepoetin alfa puede cambiar a C2S eficazmente manteniendo sus niveles de Hb.
Background: Less frequent dosing regimens during anemia treatment could benefit peritoneal dialysis (PD) patients. We investigated the effectiveness of darbepoetin alfa dosed every-other-week (Q2W) for maintaining hemoglobin (Hb) levels (11–13 g/dL). Patients and Methods: One hundred and nine PD patients from 14 centers participated in an 8-month observational, prospective study. Patients (Hb 11–13 g/dL) receiving weekly (QW) darbepoetin alfa switched to Q2W dosing at the investigator’s discretion. Doses were adjusted according to published guidelines. Results: Sixty-nine percent (75 out of 109) of patients switched to Q2W dosing. Thirty-three percent maintained the previous mean weekly dose (26.1–25.8 mg/week, equivalent to twice the previous QW dose). Forty-seven percent received a dose reduction (35.8–20.2 mg/week, equivalent to the previous QW dose). More patients in the maintenance dose group had Hb levels ³11 g/dL than those receiving a reduced weekly dose (80% vs. 51.4%, respectively, p= 0.0236). During the Q2W phase, the mean Hb level ranged from 12.0–12.5 g/dL for the maintenance dose group and 11.5–12.0 g/dL for the reduced dose group. From the switch to the end of the study, the mean (SD) change in Hb was –0.7 g/dL (0.98 g/dL, p= 0.0557) and –0.6 g/dL (1.6 g/dL, p= 0.1296) for the maintenance and reduced dose groups, respectively. The Q2W darbepoetin alfa was well tolerated. Only a single treatment-related adverse event (polycythemia) occurred. Conclusion: The majority of PD patients receiving QW darbepoetin alfa can be effectively switched to Q2W and still maintain their Hb level.
Introduction
Anemia, defined as having a hemoglobin (Hb) level < 13.5 g/dL for men and < 11.5 g/dL for women1, is a frequent and serious complication of chronic renal failure (CRF). Its main cause is an insufficient erythropoietin (EPO) level2. Low Hb levels are associated with cardiovascular disease, which in turn, is linked to a reduced survival in CRF patients3. Peritoneal dialysis (PD) is used in early-stage CRF, and although PD patients generally require fewer red blood cell transfusions than those on hemodialysis, anemia is still associated with hospitalization and mortality in this group of patients4. Hence, optimal management of anemia in PD patients is a critical clinical goal5.
European Best Practice Guidelines for the Management of Anaemia in Patients with CRF recommend treating renal anemia by supplementation of endogenous EPO, thereby improving the quality of life and reducing patient morbidity and mortality1. Darbepoetin alfa is a super-sialylated analogue of recombinant human EPO (rHuEPO), which has a longer circulating half-life than the native or recombinant hormone, and consequently, can be administered less frequently6.
An extended dosing interval results in fewer hospital visits for PD patients than for hemodialysis (HD) patients, which benefits the patients, caregivers, and physicians. Furthermore, darbepoetin alfa is administered subcutaneously, which is more convenient than the intravenous administration for PD patients who do not have a fistula.
Adult7–9 and pediatric10 PD patients have been treated successfully with darbepoetin alfa. Effective maintenance of normal Hb levels was achieved in patients who switched from once a week (QW) rHuEPO to every-other-week (Q2W) darbepoetin alfa7,9,11,12. Furthermore, in a small prospective study of PD patients (n = 11), monthly darbepoetin alfa was shown to maintain normal Hb levels13.
The primary aim of this trial was to evaluate the effectiveness of switching from QW to Q2W subcutaneous administration of darbepoetin alfa for maintaining an Hb level between 11 and 13 g/dL in patients receiving continuous ambulatory peritoneal dialysis (CAPD). The secondary aim was to evaluate the safety and tolerability of Q2W darbepoetin alfa.
Subjects and methods
Subjects
Patient inclusion criteria were: age >18 years, chronic renal insufficiency (CRI)-associated anemia, CAPD for ³ 4 months, QW darbepoetin alfa for ³ 4 months, Hb level = 11–13 g/dL, serum ferritin level ³ 100 µg/L, and serum transferrin level ³ 20%.
Key exclusion criteria were: a blood transfusion in the previous 2 months, uncontrolled high blood pressure, systemic hematological disease, major surgery within the last 8 weeks, anticipated or planned kidney transplant, pregnancy or no contraception for women of child-bearing age, and any disease contraindicative for the use of darbepoetin alfa.
The trial was conducted according to the latest revision of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients gave written informed consent. Local independent ethics committees approved the trial.
Study drug
The darbepoetin alfa used was supplied by Amgen Inc. (Thousand Oaks, CA, USA) as an injectable solution in low-volume, pre-filled, ready-to-use syringes containing 10, 15, 30, 40, 50, 60, 80, 100, 150 or 300 mg of darbepoetin alfa.
Trial design and treatment plan
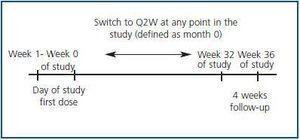
This trial was an 8-month, open-label, observational, prospective, multicenter study conducted at 14 sites in Spain (Fig. 1).Subject eligibility was assessed during the week prior to the trial. At the start of the trial (Day 1, Week 0), the patients were given subcutaneous darbepoetin alfa QW at their previously prescribed dose. The patients were then treated for a further 32 weeks. During this period, the frequency of darbepoetin alfa administration was decreased for some patients from QW to Q2W at the investigator’s discretion, based on their usual clinical practice and on the stability of the patient’s Hb level. The month that the switch occurred was defined as Month 0 for this subgroup. The Q2W dosing period was at least 4 months. During this phase, the darbepoetin alfa dose was altered according to published guidelines14 to maintain Hb levels within 11–13 g/dL. All the patients, including those who ended the trial prematurely, underwent a 4-week follow-up after the final dose of the study medication.
Effectiveness and safety assessments
Hb levels were assessed at the baseline, once every 4 weeks during the study phase (Weeks 1–32), and at the end of the trial. However, during dose and/or frequency adjustments, and during the final follow-up phase (Weeks 33–36), the Hb levels were measured every 2 weeks. The serum ferritin level, serum transferrin level, Kt/V, blood pressure, and body weight were assessed every 8 weeks during the study phase.
All patients were monitored weekly for adverse events (AEs), which were coded according to the MedDRA dictionary, and the number of patients who exceeded the 14 g/dL Hb limit was recorded.
Statistical analysis
Wilcoxon–Mann–Whitney or Student’s t-tests were used, according to data normality, to identify baseline differences between populations of patients that switched from the QW treatment to the Q2W treatment, and those who received only the QW treatment. Chi-square tests were used to compare the proportion of patients in which the weekly dose was maintained versus the proportion of patients in which the weekly dose was reduced.
Results Patient disposition
We enrolled 118 patients in the trial. Nine patients were excluded from the trial: eight patients who did not fulfill the inclusion criteria (two had not received darbepoetin alfa, three had not received QW treatment, and three had serum ferritin levels < 90 ng/mL), and one patient withdrew after the baseline visit. One patient with a baseline Hb level of 10.6 g/dL was included in the trial, as this value was considered a minor violation of the inclusion criteria.
Of the 109 patients whose data was analyzed, 75 (68.8%) changed to Q2W dosing and 28 (25.7%) maintained QW dosing. Six patients (5.5%) received darbepoetin alfa once every 10 days, and were excluded from the efficacy analysis.
Eighty-three patients (76.1%) completed the trial. Four patients withdrew because of protocol violations, and four patients discontinued the treatment because of illnesses unrelated to the treatment administered during the study. Of the remaining 18 patients, 11 received kidney transplants, three died of disease progression, two switched to hemodialysis, one failed to respond to treatment, and one was lost to follow-up.
Of the 26 patients that withdrew from the study, 10 were from the Q2W group (six in the reduced dose group, two in the maintenance dose group, one in the increased dose group, and one in the unknown dose group), and 16 were from the QW group.
Because of the observational nature of the study, data were not available for all times and patients. Consequently, the number of patients for which full data sets were available decreased over time.
Patient demographics and characteristics
Table 1 shows the baseline patient and disease characteristics. Of the total population, 59.6% of patients (65 out of 109) had been treated with rHuEPO (mean weekly dose = 5541 IU, epoetin alfa, n = 48 (77.4%), epoetin beta, n = 14 (22.6%), and no data available, n = 3) prior to darbepoetin alfa treatment.
Some baseline characteristics were significantly different between the Q2W and QW patients. Compared with QW patients, Q2W patients had a higher baseline Hb level (p = 0.004) and a higher hematocrit (36.7% versus 35.4%, respectively, p = 0.009). Q2W patients received lower baseline doses of darbepoetin alfa than QW patients did (Table 1), but the difference was not significant. Consequently, the darbepoetin alfa resistance index (baseline darbepoetin alfa dose (mg/kg/week) ´ 200 / Hb value in Month 1) was lower in the Q2W group than in the QW group (6.96 versus 9.57, respectively, p = 0.0055]. There were no other differences in baseline characteristics between the two groups.
Effectiveness
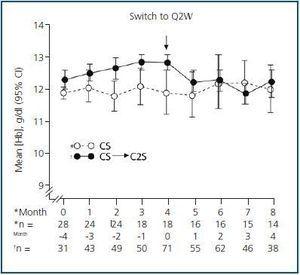
In patients that remained on QW dosing throughout the trial, the mean Hb concentration was maintained between 11 and 13 g/dL (Fig. 2). At Week 36, the mean Hb level was 11.9 g/dL (95% CI = 11.2 g/dL, 12.7 g/dL) and the mean change from the baseline was +0.12 g/dL (95% CI = –0.7 g/dL, 1.0 g/dL) (p = 0.758, n = 14).
In the cohort of patients who converted to Q2W, the mean Hb level during the QW and Q2W phases was also within the target range (Fig. 2). At the end of the trial, the mean Hb level was 12.1 g/dL (95% CI = 11.7 g/dL, 12.5 g/dL). During the Q2W phase, the mean change (95% CI) from the baseline Hb level (Month 0) at 1, 2, 3, and 4 months was –0.5 g/dL (–0.8 g/dL, –0.2 g/dL, p = 0.0006), –0.6 g/dL (–0.9 g/dL, –0.3 g/dL, p = 0.0004), –0.8 g/dL (–1.2 g/dL, –0.4 g/dL, p = 0.0001), and –0.6 g/dL (–1.1 g/dL, –0.2 g/dL, p = 0.01), respectively.
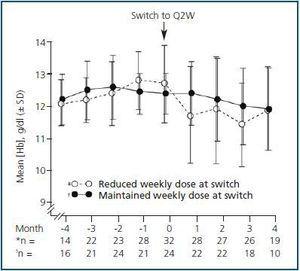
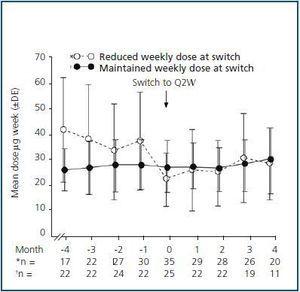
Subset analysis of the Q2W population showed that patients who received the same weekly darbepoetin dose after switching (i.e., the maintenance group) had more stable Hb levels than patients who received a reduced dose after switching did (Fig. 3). The mean Hb levels ranged from 12.0 g/dL to 12.5 g/dL for Q2W patients whose weekly dose was maintained after switching. For these patients, the mean change (SD) relative to the baseline Hb level after switching to Q2W was –0.1 g/dL (0.79 g/dL, p = 0.4089), –0.4 g/dL (0.96 g/dL, p = 0.0469), –0.4 g/dL (0.92 g/dL, p = 0.2450), and –0.7 g/dL (0.98 g/dL, p = 0.0557) at Months 1, 2, 3, and 4, respectively.
All mean post-switch Hb concentrations were lower for patients who received a reduced weekly dose of darbepoetin alfa after switching (11.8, 12.0, 11.5, and 12.0 g/dL at Months 1, 2, 3, and 4, respectively) compared with a mean value at Month 0 of 12.8 g/dL. For these patients, the mean change (SD) in Hb level relative to the baseline was –0.7 g/dL (1.1 g/dL, p = 0.0025), –0.7 g/dL (1.42 g/dL, p = 0.0285), –1.2 g/dL (1.52 g/dL, p = 0.0009), and –0.6 g/dL (1.6 g/dL, p = 0.1296) at Months 1, 2, 3, and 4, respectively.
A significantly higher percentage of patients in the maintenance group maintained Hb levels ³ 11 g/dL at Month 4 after switching (80%) compared with those who received a reduced weekly dose (51.4%) (p = 0.0236). The percentage of patients with Hb levels between 11 and 13 g/dL was 44% for the maintenance group and 20% for the group who received a reduced weekly dose (p = 0.0455).
The mean (SD) serum ferritin levels were statistically elevated (but not elevated in the clinical sense) at the end of the trial relative to the basal values (QW patients, 268.3 ± 133.9 ng/mL to 410.7 ± 245.2ng/mL, p = 0.0121; Q2W patients, 309.9 ± 196.3 ng/mL to 387.5 ± 267.5ng/mL, p = 0.0265). No significant changes in transferrin saturation were observed.
Dosing of darbepoetin alfa
The mean weekly dose of darbepoetin alfa for the QW patients varied between 39.0 mg/week and 46.5 mg/week during the 8-month period. Overall, for patients who changed to Q2W dosing, the mean weekly dose was reduced from 31.5 mg/week to 21.7 mg/week at the switch. During Month 3, the mean dose was increased to 28 mg/week to maintain the Hb level (11.8 g/dL), and then reduced to 23.9 mg/week after the expected increase in Hb concentration during Month 4 (Hb = 12.1 g/dL).
At the switch, 25 patients (33%) were maintained on the previous mean weekly dose, i.e., 26.1–25.8 mg/week. Thirty-five patients (47%) had their previous weekly dose reduced (from a mean of 35.8 mg/week to a mean of 20.2 mg/week). Only two patients (2.7%) received an increased weekly dose of darbepoetin alfa (from a mean of 40 mg/week to a mean of 52.5 mg/week). Dose data were unavailable for 13 patients (17.3%). Patients who maintained their weekly darbepoetin alfa dose received fewer dose changes post-switch (doses at Months 1, 2, 3, and 4 were 25.9, 23.9, 26.3, and 27.3 mg/week, respectively) than the group in which the weekly dose was reduced (doses at Months 1, 2, 3, and 4 were 23.9, 22.8, 28.5, and 26.3 mg/week, respectively) (Fig. 4).
Adverse events
Darbepoetin alfa was well tolerated during the study. The AEs observed during the study were mostly related to the underlying disease, and were consistent with those expected for this patient population. Of the enrolled patients (n = 118), 38 (32.2%) had AEs. Eighteen patients (15.3%) had serious AEs. The most frequently observed AE was peritonitis (9.3%). However, only one patient (0.9%) experienced a treatment-related AE (polycythemia). None of the patients withdrew from the trial because of an AE.
Discussion
Treatment of renal anemia with erythropoiesis-stimulating agents (ESAs) is common clinical practice for CRF patients on dialysis, and guidelines on their use have been published1,15. However, data is needed to optimize the clinical and economic benefits of these agents for this ever-expanding population.
As there is a paucity of data on the effect of ESAs in environments other than in clinical trials, we consider the results of our observational trial, which was conducted in a routine clinical environment, worthy of note. Our trial demonstrated that Q2W administration of darbepoetin alfa to CAPD patients is an effective and well-tolerated treatment for anemia. Similar results were observed for the QW and Q2W treatment groups. Furthermore, the dosing frequency was reduced, and only a slight increase in the dose was required to maintain target Hb levels.
As 25% of the enrolled patients were considered unsuitable for Q2W dosing with darbepoetin alfa, QW dosing was maintained for these patients. The results from this group confirm that QW dosing effectively maintains target Hb levels in CAPD patients for a minimum of 8 months without a change in dose. Interestingly, the patients that remained on QW dosing throughout the study were receiving a higher weekly dose of darbepoetin alfa at baseline than patients who changed to Q2W dosing did (42.7 mg/week versus 30.0 mg/week, respectively). This indicates that the first ones were more resistant to DA treatment.
Overall, switching from QW to Q2W darbepoetin alfa dosing maintained the mean Hb level between 11 and 13 g/dL during the 4-month evaluation phase. However, the Hb levels fluctuated, and tended to decrease slightly post-switch (range = 11.8–12.2 g/dL) compared with the QW dosing pre-switch (range = 12.2–12.8 g/dL).
Further analysis of the Q2W population revealed two distinct patient groups. At the dosing switch, 35 out of 75 patients (47%) had their weekly dose of darbepoetin alfa reduced by almost half (i.e., the Q2W dose was equivalent to the previous QW dose). Only one-third of patients (25 out of 75) maintained their previous mean weekly dose (i.e., the Q2W dose was equivalent to twice the previous QW dose). Interestingly, patients who received a reduced dose after switching were receiving a higher dose of darbepoetin alpha before the switch than patients whose weekly dose was maintained did (35 ± 19 mg/week (reduced dose group) versus 25 ± 20 mg/week (maintenance group)).
Q2W dosing equivalent to twice the previous QW dose resulted in more stable Hb levels (80% of patients in the maintenance dose group maintained Hb levels ³ 11 g/dL at Month 4 after conversion, compared with 51.4% of patients in the reduced weekly dose group), and required less frequent and smaller dose adjustments to maintain the target Hb level. In contrast, patients who had their weekly dose reduced at switching required dose increases to ensure maintenance of the target Hb level. Therefore, it is important to maintain a weekly dose (Q2W dose equivalent to twice the previous QW dose) when switching from QW to Q2W.
The majority of AEs were associated with kidney disease and its treatment. Only one event (polycythemia) was caused by darbepoetin alfa. Thus, this agent is well tolerated for Q2W dosing in CAPD patients.
A reduction in the frequency of ESA administration is convenient, and improves the patient’s quality of life. Consequently, extended dosing intervals may improve compliance. The results of our trial confirm previous reports showing that Q2W dosing with darbepoetin alfa is effective for PD patients. For example, switching PD patients on stable QW rHuEPO dosing to Q2W darbepoetin alfa maintains target Hb levels7,11,12. Self-administered epoetin beta is effective for maintaining therapeutic Hb levels during the switch from QW to Q2W administration16. However, in this trial, a small, non-statistically-significant increase in the epoetin dose was required to maintain the target Hb level (10–12 g/dL) in approximately half of the PD patients. The equivalence of QW and Q2W epoetin beta dosing in PD patients has been questioned17. Less frequent administration of ESAs, including darbepoetin alfa, is desirable for PD patients who self-administer ESAs.
The results of this study support the practical use of subcutaneous Q2W darbepoetin alfa for CAPD patients. The majority of PD patients who had stable Hb levels with QW darbepoetin alfa were effectively switched to Q2W and maintained the target Hb level of 11–13 g/dL. A significantly greater percentage of patients in the group that maintained their weekly dose after switching had Hb levels ³ 11 g/dL (80%) versus those who received a reduced weekly dose (51.4%). Prospective studies on Q2W dosing with larger sample sizes and a longer follow-up duration than this trial are required to confirm these findings.
Acknowledgements
Gardiner–Caldwell Communications (GCC), Macclesfield, UK assisted in the writing of this manuscript. Funding was provided by Amgen. Other Investigators who contributed to this study were: Maria Dolores Del Pino (H. Torrecárdenas), Maria José Espigares (H. San Cecilio), Argimiro Gandara Martínez (H. Pontevedra), Gloria Rodriguez Goyanes (H. Xeral-Calde Lugo), Jesús Grande Villoria (H. Virgen de la Concha), E. Hernández (H. Rio Carrión), Mercedes Moreiras Plaza (H. Xeral Cies Vigo), Mª del Castillo Paez (H. Virgen Macarena), Vicente Pérez Bañasco (H. Jaén), Cristina Pérez Melón (C.H.U. Ourense), Daniel Torán (H.G.S.S. Jerez), and Felipe Tejuca (H. Puerta del Mar).
Figure 1.
Table 1. Baseline patient demographics and disease characteristics
Figure 2.
Figure 3.
Figure 4.