Antecedentes: El retraso en la función del injerto (RFI) es uno de los problemas más frecuentes en las primeras semanas del trasplante renal, afectando a su evolución. Conocer los factores de riesgo de RFI puede ayudar a reducir su incidencia. Las alteraciones en los niveles séricos de calcio, fósforo y PTH son muy frecuentes en los pacientes en lista de espera de trasplante y podrían favorecer la aparición de RFI. Sin embargo, diversos estudios que han analizado la relación entre los niveles pretrasplante de calcio, fósforo y PTH y el desarrollo de RFI han obtenido resultados dispares que no permiten confirmar ni descartar que influyan en el mismo Métodos: Estudiamos los valores pretrasplante de calcio, fósforo y PTH en 449 pacientes trasplantados renales realizados entre 1994 y 2007. Se definió retraso en la función del injerto (RFI) en aquellos pacientes que precisaron diálisis durante la primera semana postrasplante. Se recogieron de las historias clínicas los datos clínicos y analíticos relacionados con RFI. Resultados: 27.3% presentaron RFI. Los factores significativos de riesgo para desarrollar RFI fueron la edad del receptor, el tipo y la necesidad de tratamiento sustitutivo renal, el título de anticuerpos anti-HLA máximos, el número de trasfusiones pretrasplante y la edad del donante. No detectamos diferencias significativas en los valores medios de calcio (9.4 ± 1.0 vs. 9.5 ± 0.9 mg/dl, p = 0.667), fósforo (5.7 ± 1.8 vs. 5.5 ± 1.5 mg/dl, p = 0.457), producto fosfo-cálcico (53.5 ± 17.2 vs. 51.8 ± 14.6 mg²/dl², p = 0.413) y PTHi (315 ± 312 vs. 340 ± 350 pg/ml, p = 0.530) en los pacientes con y sin RFI. Conclusiones: En nuestro estudio los parámetros séricos pretrasplante del metabolismo óseo-mineral no favorecen el desarrollo de RFI.
Background: abnormalities in serum calcium, phosphate, and Parathyroid Hormone (PTH) concentrations are common in patients with chronic kidney disease and have been associated with increased morbidity and mortality. One of the most common problems in the first weeks after renal transplantation is Delayed Graft Function (DGF). There are several well-known risk factors for DGF development, but the role of calciumphosphate- PTH homeostasis as a risk factor for early graft dysfunction is controversial. This issue was addressed in the current study. Methods: Pretransplant PTH, calcium and phosphate values were gathered in 449 patients that received a renal transplant in our center between 1994 and 2007. Other variables expected to influence the risk for delayed graft function were included from the clinical charts. Results: The incidence of DGF was 27.3%. DGF development was significantly associated with recipient age, type and need of renal replacement therapy, peak panel reactive antibodies, transfusion number and donor age. There were no significant differences in the mean pretransplant values of calcium (9.4 ± 1.0 vs. 9.5 ± 0.9 mg/dl, p = 0.667), phosphate (5.7 ± 1.8 vs. 5.5 ± 1.5 mg/dl, p = 0.457), calcium-phosphate product (53.5 ± 17.2 vs. 51.8 ± 14.6 mg2/dl2, p = 0.413) and PTH (315 ± 312 vs. 340 ± 350 pg/ml, p = 0.530) between patients with and without DGF. Conclusions: In our study population pretransplant serum PTH, calcium and phosphorus levels have no influence on the risk for DGF.
INTRODUCTION
Together with acute rejection, delayed graft function (DGF) is one of the most frequently occurring problems (10-50% in different series) in the initial phase of renal transplantation. The appearance of DGF affects both the graft and the patient, prolonging the hospital stay, increasing transplantation costs, predisposing to the occurrence of acute rejection and reducing the survival of the graft in the long term.1 The use of marginal donors, including non-heart beating, the elderly, hypertensive or diabetic donors has helped focus our attention on the relevance of DGF and the recognition of the risk factors which predispose to it.2 The many techniques for extraction, preservation and implantation of renal grafts as well as the various changes in recipients and donors can contribute to the development of DGF.3,4 Detailed knowledge of these risk factors can help to reduce the incidence of DGF.
Changes in the levels of serum calcium, phosphorus and Parathyroid Hormone (PTH) occur most frequently in patients with chronic kidney disease (CKD) and have been linked to an increase in cardiovascular calcification, the mortality and morbidity of these patients forming part of the systemic syndrome known as “mineral and bone disorders associated with chronic kidney disease” (MBD-CKD).5,6 Despite the implementation of international clinical guidelines, a high proportion of our patients did not reach the recommended values. Thus, up to 45% of patients in phase 5 presented with PTH values above 300pg/ml, 44.4% calcium value greater than 9.5 mg/dl, and 28% phosphorus level greater than 5.5mg/dl.7
In kidney transplant patients, isolated cases have been published on the deterioration of the graft function with intrarenal calcium deposits, related to hypercalcaemia, hyperphosphatemia and hyperparathyroidism who have improved after parathyroidectomy.8-12 However, analysis of series of patients examining the relationship between pretransplant levels of calcium, phosphorus and PTH and the development of DGF have shown conflicting results which do not confirm or refute the role of bone and mineral metabolism parameters on the initial renal graft function.13-21 The high prevalence of these disorders in the population at stage 5 (patients on the waiting list for renal transplantation) and the growing range of therapies allowing improved metabolic control, makes it interesting to study the possible role of abnormal serum pre-transplant bone-mineral metabolism in the initial graft function.
MATERIAL AND METHODS
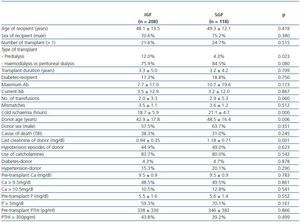
We studied the pre-transplant values of calcium, phosphorous and PTH in 449 renal transplant patients between 1994 and 2007. Patients who lost the renal graft in the first five days due to vascular thrombosis were excluded from the study. The following parameters from the prospective data base were taken: number of transplant patients, sex and age of the recipient, the type and duration of Renal Replacement Therapy (RRT), the presence of diabetes mellitus in the recipient, maximum and current antibodies and anti-HLA, number of mismatches, duration of cold ischaemia, creatinine on the fifth day after transplantation, sex and age of donor, cause of death, final donor creatinine, presence of hypotension and use of catecholamine in donor, diagnosis of diabetes or hypertension in the donor, initial immunosuppressive treatment, development of acute rejection during the first year, creatinine at six months and at one year and the duration of the renal graft. DGF was defined in those patients who needed dialysis during the first week after transplantation and Slow Graft Function (SGF) was defined in those patients with creatinine greater than 3mg/dl on the fifth day after transplantation. The rest of the patients, who did not need dialysis in the first week and whose creatinine at the fifth day was less than 3mg/dl, were included in the Initial Graft Function group (IGF).
In the pre-transplantation blood serum samples obtained routinely, the following bone-mineral metabolic parameters were obtained: calcium, phosphorus, intact PTH and pretransplant calcium x phosphate product. The total calcium values were analysed before and after correcting for albumin concentration, and there were no significant differences (data not shown). It has also been reported that in patients with chronic kidney disease, the total albumin corrected calcium values do not estimate ionic calcium better than those uncorrected.22
The intact PTH was determined using immunoradiometric assay and the calcium and phosphorus values in a standard autoanalyser. The values of calcium, phosphorus, calcium x phosphate product and intact PTH are put into variable categories, taking into account the recommended serum values following the K-DOQI and SEN guidelines (Ca > 9.5mg/dl, p > 5mg/dl, Ca x p > 55mg?/dl?, PTHi > 300pg/ml).6
The continuous variables were analysed with Student-t test and categorical variables using Chi-square. Survival analysis was performed using the Kaplan-Meier method comparing groups with the log-rank test. Significant values were considered from p < 0.05. Statistical analysis was performed with SPSS version 12.0 (SPSS Inc, Chicago Ill, USA).
RESULTS
Of the 449 patients analysed, the incidence of DGF was 123 (27.3%), 118 (26.2%) with SGF and 208 (46.3%) with IGF. The majority of the patients received immunosupressor treatment with calcineurin inhibitors (95.2%, 46.1% tacrolimus and 49.1% cyclosporine) and purine synthesis inhibitors (89.6%, 33.8% azathioprine and 55.8% mycophenolate mofetil). 11.2% of patients received treatment with sirolimus, 7.2% with anti-IL2r monoclonal antibodies and 1.5% with OKT3 or polyclonals. There were no significant differences in the development of DGF or SGF between the various immunosupressor treatments.
Thirty eight point three per cent of patients had PTH values above 300pg/ml, 48.9% had calcium values greater than 9.5mg/dl and 62.8% had phosphorus levels greater than 5mg/dl.
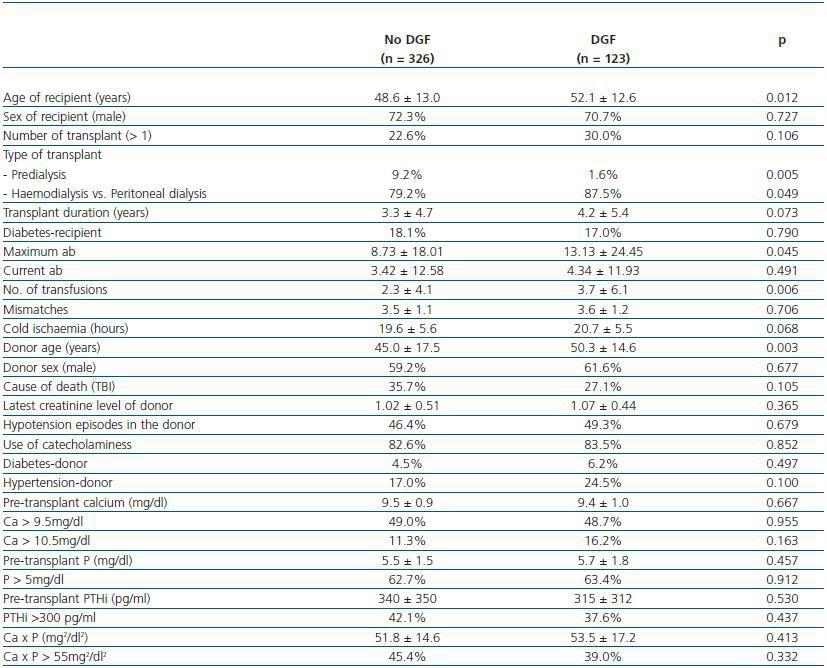
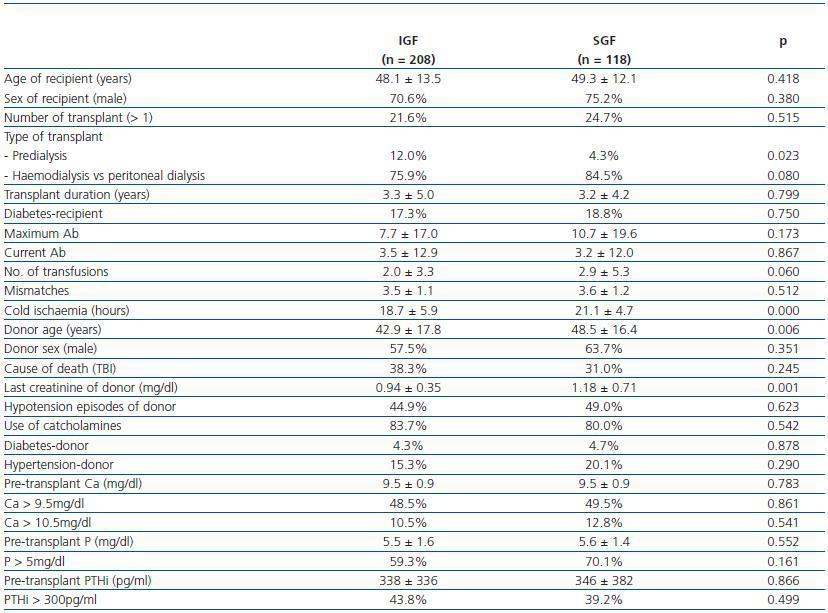
Tables 1 and 2 show the characteristics of the recipients, donors and transplant associated with the development of DGF and SGF respectively. Significant risk factors for developing DGF were the age of the recipient, the type and the need for renal replacement therapy, the peak titre of anti-HLA antibodies, the number of pre-transplant transfusions and the age of the donor (table 1). The risk factors for developing SGF were the need for renal replacement therapy, cold ischaemia, the donor age and the final donor creatinine (table 2). We did not find a relationship between the appearance of DGF or SGF and calcium levels, phosphorus, calcium x phosphate product and intact PTH.
DISCUSSION
The incidence of DGF and SGF in our series of patients is similar to those already published.3,23With respect to the risk factors of DGF, the age of both the recipient and the donor, the peak titre of pre-transplant anti-HLA antibodies and the number of transfusions are known risk factors of DGF; the last three have been included in the nomogram developed by Irish et al. to predict DGF.2,4,19,24 Humar et al. discovered from the multivariate analysis that a donor age of over 50 years and cold ischaemia for more than 24 hours were independent risk factors for suffering SGF.23 Together with these two risk factors, we find that the final creatinine level of the donor also influenced the onset of SGF.
As expected, there was a larger proportion of patients on predialysis in the group of patients who did not need renal replacement therapy during the first week. The discrepancies in the risk factors in the various published series are determined generally by the size of the study sample and by the characteristics of the suitable patients in each centre.
Against a possible beneficial role for calcium in DGF are the published isolated cases of hypercalcaemia- and hyperparathyroidism-induced DGF, the demonstration of the effect of the calcium antagonists as protectors of the DGF and the effect of intracellular calcium in experimental models of acute renal failure.
Previously published extreme cases of microscopic nephrocalcinosis in which the levels of Ca and PTH are raised much higher than usual in patients on the waiting list are not representative of the population.8
A recent meta-analysis including 13 trials with 724 participants demonstrated that the treatment with calcium channel antagonists protects against the development of DGF (RR 0.55, CI 95% 0.42-0.73), however the mechanism is not known.25 It is thought that they can reverse the vasoconstrictor effect of the calcineurin inhibitors. Furthermore, it is thought that they can intervene in the homeostasis of intracellular calcium, reducing the entry of calcium into the tubular cell and avoiding the activation of proteases which lead to cellular death.2 If the active mechanism was the latter, it would support the hypothesis that high pre-transplant calcium serum levels favour the emergence of DGF. Finally, although it is evident that calcium values above 12mg/dl induce acute renal failure by its vasoconstrictor effect and volume reduction. There is no evidence that lower serum calcium values influence the appearance of acute renal failure.2,26
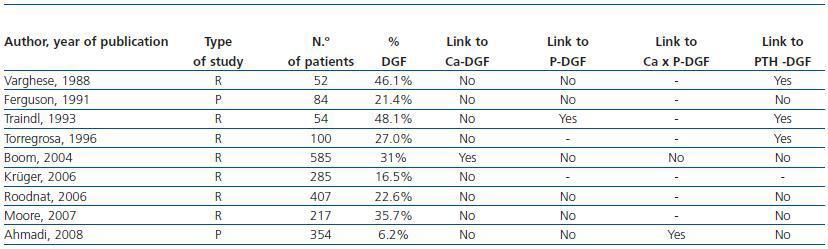
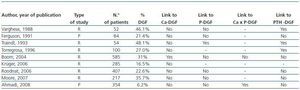
Of the nine studies which analysed how pre-transplant alterations in the calcium and phosphorus metabolism impact on the initial graft function, only the study of Boom et al found that higher values of pre-transplant calcium favour the development of DGF (table 3).13-21 Although that study has the largest number of patients (585), the rest of the studies grouped together totalled 1,553. Boom’s study also has higher mean levels of pre-transplant calcium (2.57mmol/L = 10.3mg/dl), and only calcium levels higher than 2.75mmol/L (11mg/dl) are associated with an increased occurrence of DGF (RR 2.51, CI 95%, 1.59-3.98) compared to patients with calcium lower than 2.55mmol/L (10.2mg/dl).17 By contrast, although most of the patients in our series do not meet the strict criteria of the clinical guidelines designed to optimise patient control, they show calcium values below the laboratory normal range (< 10.mg/dl), and the average calcium value was lower (9.5 ± 0.9mg/dl) and closer to the usual recommendations. In the study by Boom et al, the overlap in the pre-transplant values among patients with and without DGF was so great that it was impossible to establish a critical value above which one could predict the occurrence of DGF.17
On the other hand, it has not been shown that a relationship exists between pre-transplant calcium levels and the emergence of microscopic nephrocalcinosis underlying the DGF in the early days of transplantation. In the study by Boom et al. there was no difference in calcium levels in pretransplant patients with and without nephrocalcinosis in biopsies carried out during the first week posttransplantation. 17 It is known from the studies of serial biopsies that the proportion of biopsies with calcifications was only 6.1% in the sixth week, increasing to 17.8% at six months post-transplantation. Although calcium levels at the time of biopsy after transplantation are certainly higher in patients with nephrocalcinosis than in those who do not show it, the relationship with pre-transplant calcium levels is unknown. In addition, the occurrence of nephrocalcinosis in the sixth week is not related to the DGF.27 Therefore, one can speculate that alterations in calcium post-transplantation, not pre-transplantation, will progressively influence the appearance of calcifications in the kidney graft whereas there is no relationship between DGF and nephrocalcinosis.
In recent years, there has been an increasing number of cases and series of patients developing nephrocalcinosis and acute and even chronic kidney disease caused by phosphates, most of them after the completion of bowel cleansing prior to colonoscopy with oral sodium phosphate solution.28 In kidney transplant patients, isolated cases have also been described of graft dysfunction by calcinosis with hyperphosphataemia and hyperparathyroidism with normal calcium levels which improved after parathyroidectomy.10 In both situations very high phosphate levels led to the deterioration of kidney function. However, there is no evidence that upper limit or slightly elevated phosphate levels influence the occurrence of nephrocalcinosis and kidney damage. Of the previously mentioned studies,13-15, 17, 19-21 only one of them, with a small sample of patients analysed, showed pre-transplant phosphate levels significantly higher in patients with acute tubular necrosis (Table 3).15 In the study by Ahmadi et al. there was no significant difference in phosphate levels between patients with immediate function, SGF and DGF, while there were significant differences in calcium-phosphate product.21 In other studies, pre-transplant levels of phosphorus did not influence initial graft function.13,14,17,19,20
In all cases reported in the literature in which the alterations of calcium phosphorus metabolism have led to the initial graft dysfunction, PTH levels were elevated and the graft function improved after parathyroidectomy or a plasma exchange.8-11 Given the frequency of patients on the waiting list for transplantation with high levels of PTH, the absence of a greater number of publications with hyperparathyroidism and initial renal graft dysfunction suggests that there must be more factors which, associated with raised PTH, precipitate renal calcinosis and DGF. High PTH levels can induce a large increase in cytosolic calcium in the cells of the proximal tubule, which in turn increases the damage caused by ischaemia reperfusion injury.16 However, hyperparathyroidism is not included amongst the risk factors for acute renal failure nor has its role been studied.29 Studies in transplantation have shown that the pre-transplant levels of PTH influence initial graft function only in those with fewer patients,13-16 and with conflicting results. While three of these studies in patients with initial graft dysfunction had higher levels of pretransplant PTH, in a study by Ferguson et al, these patients had lower levels.13-16 Furthermore, none of the subsequent studies with more patients (> 200) have demonstrated a relationship between pre-transplant PTH levels and early graft function.17,19-21 In the univariate study by Roodnat et al, PTH levels influenced the DGF but its effect disappeared after adjusting for the age of the recipient and the type of donor.19
Despite not affecting the initial graft function, pre-transplant PTH levels do influence graft survival in the long term, both in uni- and multi-variate analysis.19 Patients with persistent hyperparathyroidism after transplantation tend to be those with high pre-transplant levels of PTH which, in addition, maintain a sub-optimal renal function.30 Persistent hyperparathyroidism after transplantation is associated with the onset of progressive calcification in the renal graft and kidney function worsens one year later.27 Although in our study we found that pre-transplant PTH contributes to DGF, it appears that in patients with poor kidney function after transplantation, persistent hyperparathyroidism promotes the appearance of intrarenal calcium deposits which worsen graft function.
Neither the literature nor our study can conclude that the “bone and mineral disorder associated with chronic kidney disease” contributes to the emergence of DGF. It is necessary to study more cases to detect the effect of bone-mineral metabolism in early renal transplant function. Large registry analyses of initial graft function have not included these parameters.4 In order to achieve a greater understanding, multicentre prospective studies or retrospective studies including a larger number of patients are needed.
Table 1. Comparison of characteristics between patients with and without DGF
Table 2. Comparison between patients with SGF and IGF
Table 3. Published studies on the influence of alterations of phosphocalcic metabolism in the dysfunction of the renal graft