Conservative Management (CM) has become a therapeutic option in Advanced Chronic Kidney Disease in the elderly. However, there is a lack of evidence about prognosis of these patients in terms of survival and health related quality of life (HRQoL).
ObjectiveEstablish predictive variables associated with mortality and analyse HRQoL in CM patients.
Patients and methodsProspective cohort study. An assessment of renal function parameters and a comprehensive geriatric assessment were made, including: analysis of comorbidity, functional, cognitive, fragility, nutritional, social and HRQoL status.
Results82 patients with a mean age of 84 years and significant pluripathology were studied: 56% had history of vascular event and Charlson >8. The mortality rate was 23/1000 patients per month, with a homogeneous mortality rate after 6 months.
Survival differed significantly depending on whether they presented with a previous vascular event (36.7 vs. 14.8; p=0.028), Charlson score≥10 (42 vs. 17; p=0.002), functional status (48.4 vs. 19; p=0.002) and fragility (27 vs. 10; p=0.05). Mortality predictors included eGFR and proteinuria, the presence of previous vascular events, Charlson comorbidity score, malnutrition-inflammation parameters (albumin and MNA score), degree of dependency, physical HRQoL and increase of PTH level. The presence of previous vascular event, comorbidity, decreased albumin and elevated PTH were independent predictors of mortality. HRQoL remained stable over time and no significant worsening occurred during treatment.
ConclusionsHaving knowledge of the factors associated with mortality and HRQoL assessment can be a useful tool to helping decision making during CM. Previous vascular events, comorbidity, decreased albumin and increased PTH were independent predictors of mortality.
El tratamiento renal conservador (TRC) se ha convertido en una opción terapéutica en la enfermedad renal crónica avanzada en ancianos. Se sabe poco sobre la evolución pronóstica de estos pacientes en términos de supervivencia y calidad de vida relacionada con la salud (CVRS).
ObjetivoEstablecer variables predictivas de mortalidad y analizar la CVRS en los pacientes en TRC.
Pacientes y métodosEstudio de cohortes prospectivo. Se realizó una valoración de parámetros de función renal y evaluación geriátrica integral: análisis de comorbilidad, situación funcional, cognitiva, fragilidad, nutricional, social y CVRS.
ResultadosSe evaluaron 82 pacientes, con una edad media de 84 años e importante pluripatología: el 56% tenía antecedentes de evento vascular y Charlson >8. La tasa de mortalidad fue de 23/1.000 pacientes-mes, con un ritmo de mortalidad homogéneo a partir de los 6 meses.
La supervivencia difirió significativamente si presentaban evento vascular previo (36,7 vs. 14,8; p=0,028), Charlson≥10 (42 vs. 17; p=0,002), grado de dependencia (48,4 vs. 19; p=0,002) y fragilidad (27 vs. 10; p=0,05).
Fueron predictores de mortalidad: eFG y proteinuria, presencia de evento vascular previo, comorbilidad de Charlson, parámetros de malnutrición-inflamación (albúmina y puntuación MNA), grado de dependencia, CVRS física y aumento de PTH. La presencia de evento vascular previo, comorbilidad, albúmina descendida y elevación de PTH fueron predictores independientes de mortalidad. La CVRS se mantuvo estable y no se produjo empeoramiento significativo durante el tratamiento.
ConclusionesEl conocimiento de los factores asociados con mortalidad y la evaluación de la CVRS puede ser útil como herramienta en la toma de decisiones en TRC. La presencia de evento vascular previo, comorbilidad, albúmina disminuida y el aumento de PTH fueron predictores independientes de mortalidad.
The prevalence of chronic kidney disease (CKD) in Spain has increased by 20% in the last ten years and it increases progressively with age.1 According to data from the 2016 Spanish Registry of Renal Patients, the prevalence of CKD in people aged over 64 is 25% and it is 22% in those older than 75 years.2
In addition to the ageing of the population, high prevalent diseases such as hypertension (HTN), diabetes and cardiovascular disease are contribute to the increase in CKD3; this means that it tends to occur in patients with significant comorbidity.
We are therefore increasingly faced with the clinical decision of whether to start renal replacement therapy (RRT) or conservative kidney management (CKM), having to weigh up the benefits of each intervention, in older patients with high comorbidity.4
Although overall survival tends to be higher in patients who undergo dialysis versus those who do not, the advantage is lost in patients over 80 with a greater comorbidity, especially if patients have coronary heart disease.5,6 In addition, the burden of symptoms (pain, fatigue, anorexia, dyspnoea) that these patients have on dialysis is high,7,8 their health-related quality of life (HRQoL) is often deficient,9,10 and many have progressive secondary functional deterioration.11
In view of the evident limitations of RRT for these patients, there is currently increasing interest in determining whether CKM or active management without dialysis of advanced CKD (ACKD) is a valid therapeutic option.
CKM is a comprehensive way of managing the patient which should include: interventions to delay the progression of kidney disease and minimise the risk of adverse events; shared decision making; active management of symptoms; detailed communication; advanced care planning; psychological support; and social, family and spiritual support.12
It can be difficult for us, as nephrologists, to initiate these conversations,13 in part due to the lack of prognostic information to help us advise our patients in the decision-making process.
The majority of studies report that patients on CKM have a worse prognosis, shorter survival14,15 and reduced HRQoL. Most of these studies are cross-sectional and retrospective.16
In order to provide information about the utility of CKM to patients and healthcare professionals and facilitate the process of shared decision making, we carried out a detailed analysis of patients choosing CKM. Our aims were:
- 1.
To identify variables predictive of mortality in patients opting for CKM.
- 2.
To analyse the changes in HRQoL in this population on conservative management.
This was a prospective, observational, analytical cohort study of patients seen at the Nephrology Department of the Hospital Universitario Miguel Servet [Miguel Servet University Hospital] in Zaragoza.
Inclusion criteriaOver the age of 75, incident patients on CKM.
The decision-making process was carried out by the nephrologist responsible for the patient, taking into account the following criteria:
- –
Diagnosis of ACKD with glomerular filtration rate (GFR) of less than 20ml/min/1.73m2 (in 2 separate laboratory tests 3 months apart).
- –
Patients in whom there was no clinical indication of active replacement treatment by their treating physician.
Patients who were unable to take part in the clinical interview and those who had no family support were not included.
Follow-upThe recruitment took place from 1 January 2015 to 1 May 2017. The follow-up concluded with the death of the patient or with the date of closure of the study on 31 December 2017. Scheduled visits were made at 0, 12 and 24 months of follow-up, with collection of analytical values and specific tests that analyse different areas of health: comorbidity, functional, cognitive, nutritional and social status, frailty and HRQoL.
Data sourcesThe assessments were made by the research team by conducting a clinical interview. The personal history, analytical data and date and cause of death were collected from the patient's hospital medical records and electronic medical record.
Ethical considerationsThe study was approved by the Comité Ético de Investigación Clínica de Aragón (CEICA) [Aragon Independent Ethics Committee] and all patients signed an informed consent form before they were included in the study.
Recorded variables- –
Demographic variables: age and gender.
- –
Clinical variables:
- •
Weight and height with calculation of body mass index (BMI): weight(kg)/height(m)2.
- •
Previous medical history: HTN, diabetes, cause of CKD, previous vascular event (history of coronary heart disease, cerebrovascular disease or peripheral vascular disease).
- •
Comorbidity: Charlson index.
- •
Functional status: Barthel index.
- •
Cognitive status: Pfeiffer test.
- •
Frailty: FRAIL Questionnaire.
- •
Nutritional assessment: Mini-Nutritional Assessment Short-Form.
- •
CVRS: The SF-36 Health Survey Questionnaire v2.
- •
Social status: Gijón scale.
- –
Analytical variables: eGFR (CKD-EPI ml/min/1.73m2), Proteinuria (g/l), Albumin (mg/dl), High-sensitivity C-reactive protein ([hs-CRP] mg/dl), NT-proBNP (pg/ml), Potassium (mEq/l), Haemoglobin (g/l), PTH (pg/ml), Calcium (mg/dl) and Phosphorus (mg/dl).
- –
Comorbidity was classified using the Charlson index adjusted to age,17,18 according to the definitions established in the original article published in 1987, and adding one point for each decade over the age of 50 to the value obtained. Scores of 6–7 are considered high and scores of 8 or over very high.
- –
Functional status for basic activities of daily living was assessed using the Barthel index.18–20 This classifies patients as independent (score 91–100), minor dependency (61–90), moderate dependency (41–60), severe dependency (21–40) or total dependency (20 points or below).
- –
Cognitive assessment was performed using the Pfeiffer test.18,21 This is a rapid screening test in which a score of three points or higher indicates that there may be cognitive impairment.
- –
To identify frailty, we used the FRAIL Questionnaire.22 This consists of five simple questions related to fatigue, resistance, ambulation, illness, and loss of weight; each is scored with one point. Unlike the Fried frailty criteria, it does not require a dynamometer to assess muscle strength, and like those criteria, patients are classified as frail when they score 3–5 points.
- –
The Mini-Nutritional Assessment Short-Form test (MNA-SF) was used to assess nutritional status.23 The MNA is a validated screening tool that helps identify older people who are malnourished or at risk of malnutrition. A total score of 12 or above indicates that the person is well nourished, from 8 to 11 reflects that the person is at risk of malnutrition, and 7 or below means that the person is malnourished.
- –
Social status was identified through the Gijón Socio-family Scale (Barcelona abbreviated version)24: a score of less than 7 points indicates a good social situation; between 8 and 9 points, intermediate situation; and more than 10 points, social deterioration.
- –
Quality of life was assessed using the 36-item Short Form (SF-36) Health Survey v2.25 This is a generic instrument for assessing HRQoL, translated into Spanish, which consists of 36 items grouped into eight dimensions: physical functioning (PF), role limitations due to physical problems (RP), bodily pain (BP), general health perception (GH), vitality (VT), social role functioning (SF), role limitations due to emotional problems (RE) and mental health (MH).
For each dimension the items are coded, added together and transformed into a scale from 0 (worst possible health state) to 100 (best possible health state). It allows the calculation of two summary scores: one on physical health status and another on mental health. The scores were obtained using QualityMetric Incorporated Health Outcomes Scoring Software 5.0.
The internal consistency (Cronbach's alpha) of the different scales ranged from 0.71 to 0.95.
Statistical methodsQuantitative variables are described with their mean and standard deviation (SD) or with their median and interquartile range (IQR), while the qualitative variables are expressed with their frequency distribution. The quantitative variables were compared with Student's t-test or the non-parametric Mann–Whitney U test. The χ2 test was used for the comparison of qualitative variables with linear trend estimation in variables with ordered categories. The statistical significance of the change in the quantitative variables at the different time points was determined with Friedman's non-parametric test.
The total number of deaths was considered as primary endpoint. Patients were followed up from their inclusion until they left the study because the study closure date had been reached or as a result of death or loss to follow-up. Mortality rates are expressed per 1000 patient-months. We determined survival functions (with Kaplan–Meier method) and risk (evaluation of mortality dynamics). The comparison of rates between groups was made with the log rank test.
Assessment of the independent contribution of the initial variables to mortality rates was confirmed in Cox regression models, with estimation of hazard ratios (HR) and 95% confidence intervals (CI). The variables to be introduced in the multivariate models were chosen according to their clinical meaning or their statistical association in univariate analysis with p<0.1. Lastly, the best mortality prediction model was selected using the sequential exclusion procedure.
Associations with p<0.05 were considered significant. The SPSS software package version 22.0 was used.
ResultsA total of 82 patients on CKM were included: 56 patients in 2015, 23 in 2016 and three in 2017. Over the course of the follow-up, one patient was excluded from the analysis after deciding to abandon CKM and start haemodialysis and two patients because of loss to follow-up.
The causes of ACKD were: nephroangiosclerosis (57.4%); diabetic nephropathy (25.7%); chronic tubulointerstitial nephropathy (6.1%); cardiorenal syndrome (4.9%); glomerulonephritis (2.4%); and other (3.7%).
Of the 82 patients in the study, the main reason for opting for CKM was: a serious illness unlikely to improve with RRT in 61 patients; severe functional dependence in eight patients; advanced cognitive impairment in five patients; own decision in five patients; limiting psychiatric disease in two patients; and metastatic cancer in one patient.
The baseline characteristics of the sample overall and of the deceased and non-deceased patients are shown in Table 1.
Baseline characteristics of the sample overall and of the deceased and non-deceased patients.
| Whole group (n=82) | Non-deceased (n=44) | Deceased (n=38) | p | |
|---|---|---|---|---|
| Gender (male %) | 50 | 43.1 | 57.9 | 0.18 |
| Age (years) | 84.79±3.99 | 84.06±4.52 | 81.55±14.17 | 0.6 |
| HTN (%) | 98.8 | 97.7 | 100 | 0.35 |
| DM (%) | 38 | 40.9 | 52.6 | 0.28 |
| Previous vascular event % (CHD, CVA, PVD) | 56.1 | 45.5 | 68.4 | 0.03 |
| BMI (kg/m2) | 28.62±4.71 | 28.85±4.59 | 28.5±4.54 | 0.6 |
| eGFR CKD-EPI (ml/min/1.73m2) | 16.38±2.85 | 17.14±2.19 | 15.46±3.28 | 0.02 |
| Proteinuria (g/l) | 0.72±1.34 | 0.41±0.69 | 1.08±1.77 | 0.12 |
| UPCR (mg/dl) | 1.97±4.92 | 0.97±1.27 | 2.91±6.71 | 0.4 |
| ProBNP (pg/ml) | 8034.11±9000.88 | 2075.0±1280.20 | 10,588.0±9818.26 | 0.008 |
| Albumin (mg/dl) | 3.82±0.49 | 3.95±0.40 | 3.60±0.54 | 0.02 |
| PTH (pg/ml) | 223.7±148.96 | 211.04±114.42 | 239.85±184.93 | 0.8 |
| Phosphorus (mg/dl) | 3.70±0.64 | 3.70±0.59 | 3.87±0.68 | 0.3 |
| Calcium (mg/dl) | 9.39±0.63 | 9.51±0.46 | 9.24±0.78 | 0.07 |
| Potassium (mEq/l) | 4.61±0.56 | 4.73±0.69 | 4.68±0.63 | 0.7 |
| Haemoglobin (g/l) | 11.56±1.44 | 11.75±1.36 | 11.33±1.51 | 0.13 |
| Charlson Index | 8.9±1.40 | 8.6±1.29 | 9.2±1.46 | 0.06 |
| Barthel index (%) | 0.029 | |||
| Independent | 4.9 | 6.8 | 2.6 | |
| Slight dependency | 69.5 | 75 | 63.2 | |
| Moderate dependency | 13.4 | 13.6 | 13.2 | |
| Severe dependency | 2.4 | 0 | 5.3 | |
| Total dependency | 9.8 | 4.5 | 15.8 | |
| Pfeiffer more than 3 mistakes (%) | 26.3 | 20.5 | 33.3 | 0.19 |
| Frailty (%) | 78 | 68.2 | 88.5 | 0.02 |
| MNA test (%) | 0.07 | |||
| Malnutrition | 17.3 | 11.4 | 24.3 | |
| Malnutrition risk | 50.6 | 50 | 51.4 | |
| Normal nutritional status | 32.1 | 38.6 | 24.3 | |
| Gijón socio-family assessment scale (%) | 0.7 | |||
| Good | 79.3 | 81.4 | 76.3 | |
| Intermediate | 17.1 | 15.9 | 18.1 | |
| Decline | 3.7 | 1.2 | 5.3 | |
| SF-36 physical component | 37.11±8.30 | 38.9±7.67 | 34.48±8.62 | 0.02 |
| SF-36 mental component | 43.81±10.23 | 44.12±11.13 | 43.36±8.94 | 0.46 |
The mean age was 84.79±3.99 years, 50% of the patients were male and 56% had history of previous vascular event. A Charlson index score above 8 shows a high degree of comorbidity.
Analysis of mortality rates and factors predicting deathDuring the follow-up period 38 patients died: 26 (68.4%) in hospital and 12 (31.6%) at home. The causes of death were cardiovascular disorders in 15 patients (39.5%), progression of ACKD in 14 patients (36.8%), infections in four patients (10.5%), metastatic cancer in three patients (7.9%), trauma in one patient (2.6%) and unknown in one patient (2.6%).
We analysed the difference between patients who died and those who were still living after the follow-up period. Patients who died had a higher prevalence of previous vascular events, lower eGFR and albumin, and higher NT-proBNP levels. They also had a higher degree of dependence and frailty, a worse score in the physical component of the SF-36 and, at the limit of statistical significance, a poorer nutritional status and a higher Charlson comorbidity score.
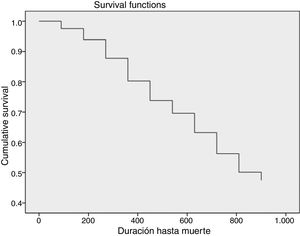
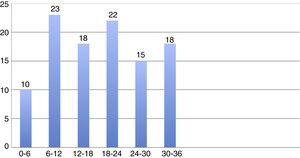
The total mortality rate was 23/1000 patient-months (Fig. 1). Fig. 2 shows the mean monthly mortality rate by semesters over the course of the follow-up; after the first six months the mortality rate remained uniform for the rest of the follow-up period.
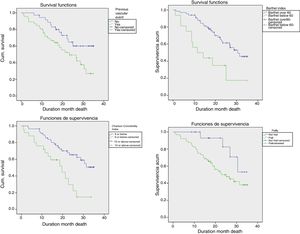
Median survival was 26.9 (95% CI: 19.6–34.2) months. There were significant differences (Fig. 3) in patient survival depending on whether or not they had a previous vascular event (mortality rate 36.7 vs 14.8, p=0.028), Charlson score ≥10 versus <10 (mortality rate 42 vs 17; p=0.002), and a high degree of functional dependence (mortality rate 48.4 vs 19; p=0.002), and whether or not they were frail (mortality rate 27 vs 10, p=0.05).
Table 2 shows the variables significantly associated with increased mortality rates by univariate analysis. The variables cognitive impairment by the Pfeiffer test and frailty were at the limit of statistical significance. Renal involvement parameters (GFR and proteinuria), a previous vascular event, the Charlson comorbidity index, malnutrition-inflammation parameters (albumin and MNA score), degree of dependency, physical quality of life, and phosphorus and calcium metabolism parameters (increase in PTH) were all predictors of mortality. In multivariate analysis, a previous vascular event, comorbidity measured by the Charlson index, descending albumin and elevation of PTH were independent predictive variables related to mortality in older patients receiving CKM.
Variables associated with higher mortality rates: univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| eGFR (ml/min/1.73m2) | 0.81 (0.72–0.91) | <0.001 | ||
| Proteinuria (g/l) | 1.50 (1.26–1.92) | <0.001 | ||
| Albumin (mg/dl) | 0.34 (0.17–0.68) | 0.002 | 0.23 (0.10–0.52) | <0.001 |
| PTH (pg/ml) | 1.002 (1.000–1.005) | 0.05 | 1.004 (1.001–1.007) | 0.007 |
| Previous vascular event (presence) | 2.11 (1.06–4.2) | 0.03 | 2.70 (1.05–6.92) | 0.028 |
| Charlson comorbidity index (points) | 1.40 (1.11–1.77) | 0.004 | 1.56 (1.14–2.12) | 0.005 |
| Barthel index (points 0–100) | 0.98 (0.97–0.99) | 0.005 | ||
| Pfeiffer (points) | 1.08 (0.99–1.19) | 0.07 | ||
| Frailty (%) | 0.37 (0.13–1.05) | 0.06 | ||
| MNA (points) | 0.86 (0.75–0.99) | 0.04 | ||
| SF-36 physical component | 0.94 (0.89–1.00) | 0.05 | ||
| SF-36 mental component | 0.99 (0.96–1.03) | 0.84 | ||
For cognitive reasons or because of physical limitations, 16 patients (19.5%) in the baseline assessment, 26 patients (41.2%) at 12 months and 12 patients (40%) at 24 months were unable to complete the test. Of the 64 patients who completed the baseline study, 37 were assessed at baseline and 12 months, and 18 at baseline, 12 and 24 months.
At the beginning of the study the physical dimensions were affected (physical functioning, role limitations due to physical problems, bodily pain and general health perception) with better preservation of mental health in the role limitations due to emotional problems and mental health dimensions (Table 3).
Analysis of health-related quality of life over the course of the conservative treatment.
| 0 months (n=64) | 12 months (n=37) | 24 months (n=18) | p Friedman | |
|---|---|---|---|---|
| Physical functioning (PF) | 40.70±31.65 | 44.60±30.03 | 47.78±31.54 | 0.63 |
| Role limitations due to physical problems (RP) | 36.04±24.31 | 37.67±24.45 | 38.89±22.23 | 0.89 |
| Bodily pain (BP) | 66.04±23.07 | 67.01±25.55 | 69.22±26.71 | 0.22 |
| General health perception (GH) | 38.51±17.51 | 41.86±14.02 | 46.44±9.12 | 0.40 |
| Vitality (VT) | 43.45±16.90 | 41.89±14.84 | 46.53±15.04 | 0.06 |
| Social functioning (SF) | 46.88±18.90 | 42.23±17.51 | 41.67±17.67 | 0.06 |
| Role limitations due to emotional problems (RE) | 58.07±29.91 | 65.55±27.85 | 75.01±25.72 | 0.006 |
| Mental health (MH) | 66.17±21.37 | 72.30±17.14 | 74.44±17.81 | 0.04 |
| Physical component (PCS) Norm=50 | 37.11±8.30 | 37.44±8.90 | 37.78±7.93 | 0.38 |
| Mental component (MCS) Norm=50 | 43.81±10.23 | 45.34±8.75 | 47.80±7.86 | 0.21 |
SF-36 v2: scores of 50 (10), the reference population has a mean of 50 with a standard deviation of 10, so values greater than or less than 50 indicate a better or worse health status, respectively.
As significant findings, the patients experienced a more marked feeling of fatigue in the first 12 months of treatment (VT 0 months 43.45±16.90 vs VT 12 months 41.89±14.84, p=0.03). They also felt restrictions in their usual social relationships throughout the 24 months of follow-up (SF 0 months 46.88±18.90 vs SF 12 months 42.23±17.51, p=0.001 and SF 0 months 46.88±18.90 vs SF 24 months 41.67±17.67, p=0.015).
However, patients reported less limitation in daily activities due to emotional problems during the treatment (RE 0 months 58.07±29.91 vs RE 24 months 75.01±25.72, p=0.05 and RE 12 months 65.55±27.85 vs RE 24 months 75.01±25.72, p=0.05) along with an improvement in perception of mental health, although that was not statistically significant.
We analysed changes in HRQoL at different points during follow-up using the Friedman test. We found that quality of life remained stable and the change in time was not significant, with improvement in the limitation in the daily activities due to emotional problems and a good perception in terms of mental health.
DiscussionWe found in our study that the patients treated with CKM were patients whose kidney disease had become chronic and who had multiple problems26 due to associated comorbidity (56% of patients had had a previous vascular event, 38% were diabetic and the mean Charlson comorbidity index score was 9). Many of these patients were complex chronic patients as, in addition to having different progressive illnesses, they were also on polypharmacy, were dependent for basic activities of daily living and had poor social status (13.4% of our patients had moderate dependence, 2.4% severe and 9.8% total, and 20% had socio-family problems). CKM should not mean “no treatment” or less specialised care. It must provide comprehensive care which aims to delay the progression of the kidney disease, manage the symptoms and, when the time comes, plan advanced palliative care.6
Our results show that the median survival in CKM patients was 26 months. Wong et al.15 reported a survival time of 23.4 months, but included patients with GFR below 30ml/min/1.73m2. Chanda et al.,27 reported mean survival of 21.2 months, Murtagh et al.,5 18 months and Ellam et al.,14 21 months, but in these studies the GFR was 15ml/min/1.73m2.
The above studies, all with similar heterogeneous inclusion criteria, analysed the survival of older people on CKM compared to RRT. The majority showed that dialysis increased survival compared to conservative management, but being over 80,28 having comorbidity27,29 and coronary heart disease5 substantially reduced these differences.
The mortality rate was consistent throughout the follow-up period. The fact that the mortality rate did not increase in a given period, particularly during the first months of treatment, and that the main causes of death were cardiovascular and progressive deterioration in kidney function underline that these patients did have the benefit of adequate follow-up and multidisciplinary care, without resorting to other more aggressive interventions.
The geriatric assessment30–33 provides an overall approach to older people through clinical assessment (with identification of chronic diseases and nutritional aspects), functional assessment, cognitive assessment, psychosocial assessment and frailty, and is becoming increasingly important in patients with CKD.18 Assessment and treatment require a multidisciplinary approach in patients with CKD, with strategies that identify factors for worse prognosis which can help in decision making.
In our study, we found that the patients with the shortest survival were those who were frail, with the greatest initial comorbidity, a previous vascular event and the greatest degree of dependence. In the univariate analysis, eGFR, proteinuria, albumin, nutritional status and increased PTH were also associated with mortality. In the final multivariate analysis, a high degree of comorbidity, a previous vascular event, a decrease in albumin and an increase in PTH all maintained statistical significance.
Although the mean albumin and PTH values in our patients were adequate, our analysis shows that the higher the albumin levels and the lower the PTH levels, the better the prognosis.
Previous studies describe comorbidity as a prognostic factor for mortality.15 Ellam et al.14 found that albumin levels above 3.5mg/dl were associated with longer survival. Joly et al.34 analysed patients over 80 on CKM and RRT and found that octogenarians who did not go on dialysis differed from those on RRT in terms of degree of dependence, comorbidity and diabetes. In the Cox analysis, the independent predictors of death on dialysis were nutritional status, functional dependence and the presence of macroangiopathy defined by peripheral vascular disease.
The prevalence of vascular calcification is documented in patients with ACKD.35 It is possible that it has a multifactorial aetiology and is due to ageing, comorbidity and mineral and bone metabolism (MBM) disorders. MBM disorders also lead to abnormal bone architecture,36 fractures37 and decreased mobility in these patients, which could explain the relationship we found between elevated PTH levels and mortality.
Frailty was not an independent predictor of mortality in our study, but the results were at the limit of statistical significance. One explanation could be the limited number of patients, which subtracts statistical power, and the superposition of comorbidity and dependence already described in previous studies in a cohort of dialysis patients.38
Most of the studies that analyse quality of life in patients with ACKD are focused on patients on dialysis, especially haemodialysis, and are of a cross-sectional observational design.9,10 We analysed the changes in HRQoL in CKM patients and our results show that the most affected areas were the physical dimensions and the difficulty with their social relationships. However, the patients perceived less limitation in daily activities due to emotional problems and had a good perception of their mental health from the point of view of a feeling of well-being and being calm.
In a recent study of survival and changes in HRQoL39 conducted with patients on CKM and RRT, physical quality of life in conservative management was sustained or improved after 12 months of treatment in less than half of the patients, but mental health was sustained or improved in somewhat more than half. Our results show that patients on CKM do perceive the physical limitations due to the passage of time and progression of their disease, but they also feel continued greater satisfaction in psychological dimensions, with a quality of life that does not worsen over time but remains stable, which is the primary aim of CKM.
As far as the contributions made by our study are concerned, we would like to emphasise that it was a prospective study which included assessment of renal function parameters, functional status, frailty, nutritional status and HRQoL. Our analysis of survival and quality of life in patients who have already made the decision to have conservative management may provide a useful tool for informing and guiding the patient in the shared decision process. We also performed a comprehensive analysis of older patients, with this being an increasingly numerous but not very visible group because of a lack of registries on conservative management.20
The limitations of our study include the lack of a comparative group, in which we could have analysed survival and quality of life in patients recommended for dialysis, and the small sample size, which reduces the statistical power of our results. We used version 2 of the SF-36 instrument which includes improvements in the metric characteristics of two dimensions, some simpler statements to facilitate reading and completion and a calculation programme which makes it easier to estimate missing responses. However, although it is translated into Spanish and its use enables greater cultural comparability, it has not been validated for the Spanish population, so we cannot compare our results with the general population in Spain.
In conclusion, in our group of patients on CKM we found the mortality rate to be high and uniform over time, independent predictors of which were a previous vascular event, comorbidity measured by the Charlson index, descending albumin levels and elevated PTH. We also found that quality of life remained stable during treatment. We therefore believe that adequate CKM could be a recommended therapeutic option in these patients, who will also receive active treatment of hyperparathyroidism and prevention and treatment of malnutrition, and autonomy and physical rehabilitation will be encouraged.
We consider necessary to have multidisciplinary teams (palliative care teams [doctors and nurses], geriatricians, nutritionists, physiotherapists and primary care) in place to provide better care in CKM patients. Also necessary is more coordination with palliative care and home support teams, especially in the later stages of the disease, in order to maintain or even improve HRQoL for these patients and their families.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Rubio Rubio MV, Lou Arnal LM, Gimeno Orna JA, Munguía Navarro P, Gutiérrez-Dalmau A, Lambán Ibor E, et al. Supervivencia y calidad de vida en pacientes ancianos en tratamiento renal conservador. Nefrologia. 2019;39:141–150.