Many European countries have transplant programs with controlled donors after cardiac death (cDCD). Twenty-two centers are part of GEODAS group. We analyzed clinical results from a nephrological perspective.
MethodsObservational, retrospective and multicentre study with systematic inclusion of all kidney transplant recipients from cDCD, following local protocols regarding extraction and immunosuppression.
ResultsA total of 335 cDCD donors (mean age 57.2 years) whose deaths were mainly due to cardiovascular events were included. Finally, 566 recipients (mean age 56.5 years; 91.9% first kidney transplant) were analyzed with a median of follow-up of 1.9 years. Induction therapy was almost universal (thymoglobulin 67.4%; simulect 32.8%) with maintenance with prednisone-MMF-tacrolimus (91.3%) or combinations with mTOR (6.5%). Mean cold ischemia time (CIT) was 12.3h. Approximately 3.4% (n=19) of recipients experienced primary non-function, essentially associated with CIT (only CIT≥14h was associated with primary non-function). Delayed graft function (DGF) was 48.8%. DGF risk factors were CIT≥14h OR 1.6, previous haemodialysis (vs. peritoneal dialysis) OR 2.1 and donor age OR 1.01 (per year). Twenty-one patients (3.7%) died with a functioning graft, with a recipient and death-censored graft survival at 2-years of 95% and 95.1%, respectively. The estimated glomerular filtration rate at one year of follow-up was 60.9ml/min.
ConclusionsCIT is a modifiable factor for improving the incidence of primary non-function in kidney transplant arising from cDCD. cDCD kidney transplant recipients have higher delayed graft function rate, but the same patient and graft survival compared to brain-dead donation in historical references. These results are convincing enough to continue fostering this type of donation.
Varios países europeos disponen de programas de donación tras parada cardiaca controlada (cDCD). Veintidós centros participan en el grupo GEODAS, cuyos resultados clínicos presentamos desde una perspectiva nefrológica.
MétodosEstudio multicéntrico retrospectivo observacional con inclusión sistemática de todos los trasplantes renales (TR) procedentes de cDCD, siguiendo protocolos locales de extracción e inmunosupresión.
ResultadosSe incluyó a 335 donantes tras cDCD (edad media 57,2 años) fallecidos mayoritariamente por eventos cardiovasculares. Se analizan 566 receptores (edad media de 56,5 años; el 91,9% con primer trasplante renal), con una mediana de seguimiento de 1,9 años. La terapia de inducción fue casi universal (timoglobulina 67,4%; simulect 32,8%) con mantenimiento con prednisona-MMF-tacrolimus (91,3%) o combinaciones con mTOR (6,5%). El tiempo medio de isquemia fría (CIT) fue 12,3h. Hubo un 3,4% de fallo primario del injerto (n=19), asociado fundamentalmente al tiempo de isquemia fría (solo el CIT ≥ 14h se asoció a fallo primario del injerto). La función retrasada del injerto (DGF) fue 48,8%. Los factores de riesgo para la DGF fueron: CIT ≥ 14h OR 1,6, procedencia de hemodiálisis (vs. diálisis peritoneal) OR 2,1 y edad del donante OR 1,01 (por año). Veintiún pacientes fallecieron con injerto funcionante (3,7%), con una supervivencia de paciente e injerto (censurada para muerte) al segundo año del 95% y del 95,1%, respectivamente. El filtrado glomerular estimado al año de seguimiento fue 60,9ml/min.
ConclusionesEl CIT es un factor modificable para mejorar la incidencia del fallo primario del injerto en trasplante renal procedente de cDCD. El trasplante renal con cDCD tiene mayor incidencia en la función retrasada del injerto, pero igual supervivencia de paciente e injerto que la referencia histórica para donación en muerte encefálica. Los resultados son satisfactorios para continuar promoviendo este tipo de donación.
Renal transplantation (RT) is the best therapeutic option of renal replacement therapy.1,2 The rate of kidney donation in 2017 in Spain was 46.9 donation per million of habitants (pmp). RT represents the first modality of renal replacement therapy and it covers a 53% of patients. This has been achieved to a large extent because the acceptance of new donor profiles. In 2011 the death brain donation (DBD) had stabilized and the 2229 RT performed was not enough for the 4524 patients remaining in the waiting list.3 At that time, it was promoted the use of organs from donors with expanded criteria and from non-controlled asystolia donation (ncDCD) aiming to improve the number of RT. The ncDCD model was launched in our country in 1992; it has a complex organization and infrastructure and, therefore, it was limited to a few hospitals.3 At the same time, several European countries had donation programs after controlled circulatory arrest (cDCD), with satisfactory results .4,5
In 2011, the Spanish National Transplant Organization (ONT) in collaboration with hospitals and scientific societies, promoted an institutional plan to stimulate the Maastricht type 3 donation, in which the donor presents a controlled circulatory death in the hospital environment (cDCD).6
In 2012, these programs were launched in several hospitals and the experiences of the first series of cases were published soon after.7 The same year, the transplant working group of the Spanish Society of Nephrology (SENTRA) promoted a cooperative study among the hospitals that had incorporated this donation model (GEODAS-3 study). This manuscript describes and analyzes the data of this national experience.8
MethodsDesignThis is a retrospective multicentre analysis of a database that includes all the TR recipients of a cDCD from the 22 participating hospitals that were incorporating patients as they started the program. The first center started in January 2012 and patients were included until December 2016. This project was approved by the Investigation Ethics Committee of the Puerta de Hierro Hospital, and has been sponsored by the transplant working group of the Spanish Society of Nephrology (SENTRA) and it is part of the REDINREN project (Work Package 6)sponsored by ISCIII 016/009/009.
Variables collectionEach center has its own process for the extraction and preservation of organs with ultrafast, rapid laparotomy with antemortem catheter placement and cold perfusion, or perfusion at normal temperature with extracorporeal membrane oxygenation (ECMO). There was not an unified immunosuppression protocol. However, following the recommendations of current clinical guidelines, monoclonal or polyclonal antibodies was used for induction. Each center has a database of identical structure for the collection of information and clinical monitoring that was completed prospectively. Periodically, in the coordination center, the data was integrated and repaired using logical routines of rank and internal consistency. We have included systematic all the cases that receive an organ from cDCD donation generated each hospital of the participating group or in an external one. Our objective was to monitor the RT performed in the hospitals included in the group; therefore, the kidneys sent to other centers were not included in the analysis.
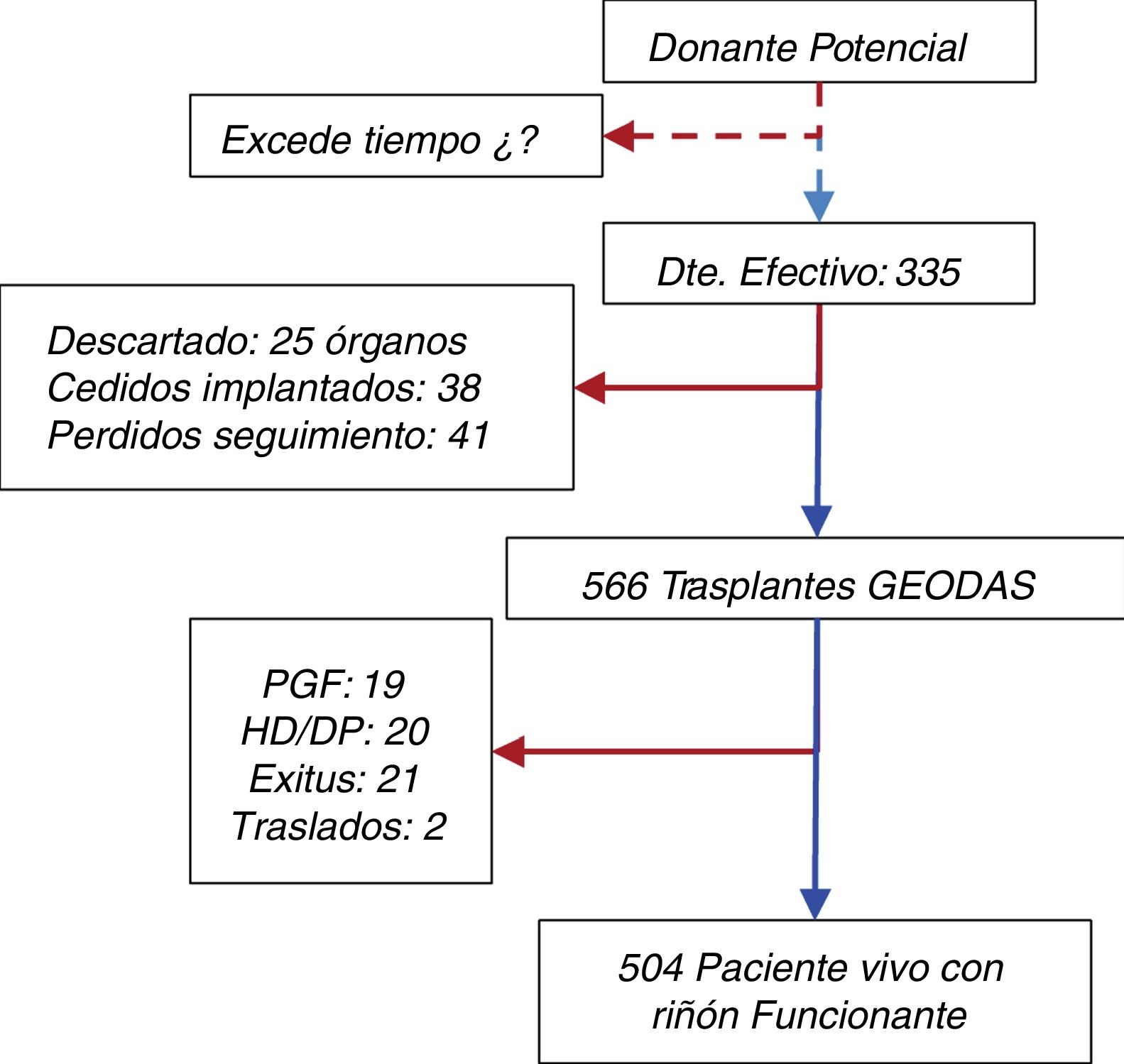
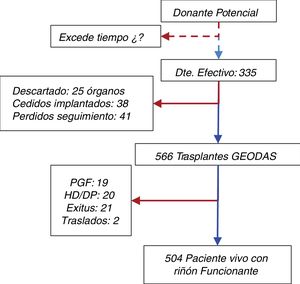
DefinitionsThe definitions of clinical events are the following: (a) primary graft failure (PNF), which is a graft that never had any function; (b) delayed graft function (DGF), that needed at least one hemodialysis session during the first week after transplantation9; (c) Graft with renal function at year 1. The glomerular filtration rate was estimated by the formula Modified Renal Diet Disease-4 (MDRD-4) and (d) survival of the patient with functioning graft and graft censored by death of the patient using Kaplan–Meier curves. Patients were followed until death or graft failure. The patient flow chart is shown in Fig. 1
Statistical analysisThe quantitative variables are shown as mean and standard deviation (SD) or median and interquartile range (IQR), depending on whether the values follow a normal distribution. Categorical variables are expressed as frequency or percentage. Comparisons between groups are made by Student's t test for comparison of means and the chi-square test for comparison of percentages. In all cases, it is considered a significant difference when p<0.05. The optimal cut-off point for the cold time of ischemia (CTI) variable was analyzed using ROC (receiver operating characteristic) curves, looking for the balance between sensitivity and specificity. The Kaplan–Meier curve was use for survival analysis. In addition, it was performed a univariate analysis using binary logistic regression to evaluate the risk factors associated with the manifestation of clinical events: PNF and DGF. The multivariate analysis includes all variables with p<0.10 value in the univariate, as well as other clinically relevant variables to control for confounding bias. The results of the models are shown as odds ratio (OR) with its 95% confidence interval. The database was designed in Access® (Microsoft Inc., USA) and the statistical analysis was performed with Stata v14 (StataCorp 2015, College Station, TX, USA).
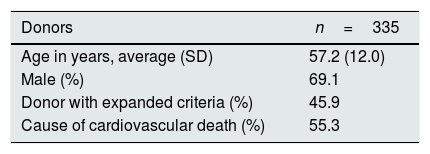
ResultsDescription of the donors and the donation processA total of 335 cDCD donors were included, mostly males (69.1%) and with an average age of 57.2 years (SD 12.0, range 2–86). The donors were admitted to the critical care units for various reasons and had reached an irreversible situation with a firm decision, by their usual clinical team, to suspend life support measures. The diseases causing death, comorbidity and serological status are summarized in Table 1. The donors had an average serum creatinine (Crs) of 0.7mg/dl (SD 0.4, range 0.3–2.1mg). Preimplantation biopsies were performed in 36.7% of the cases.
Baseline characteristics of donors, recipients and relevant data of the transplant process.
| Donors | n=335 |
|---|---|
| Age in years, average (SD) | 57.2 (12.0) |
| Male (%) | 69.1 |
| Donor with expanded criteria (%) | 45.9 |
| Cause of cardiovascular death (%) | 55.3 |
| Receptors | n=566 |
|---|---|
| Age in years, average (SD) | 56.5 (12.0) |
| Age>65 years (%) | 24.1 |
| Male (%) | 68.4 |
| Diabetes Mellitus (%) | 32.1 |
| Previous cardiovascular event (%) | 10.8 |
| Etiology of CKD(%) | |
| Glomerulonephritis | 17.5 |
| Hypertensive nephropathy | 12.2 |
| Diabetic nephropathy | 13.8 |
| Interstitial nephropathy | 11.1 |
| Polyquistosis | 14.5 |
| Other | 10.3 |
| Unknown | 20.7 |
| Previous renal therapy (%) | |
| Hemodialysis/peritoneal dialysis | 75.4/19.7 |
| Transplant in predialysis | 4.9 |
| Previous time in dialysis in years, median (IQR) | 2.02 (1.19–3.57) |
| Patients without previous transplant (%) | 91.9 |
| Characteristics of the transplant | |
|---|---|
| Number of HLA incompatibilities, average (SD) | 3.9 (1.3) |
| Cold ischemia in hours, average (SD) | 12.3 (6.5) |
| Warm ischemia in minutes, average (SD) | 26.5 (15.6) |
| Induction (thyroglobulin/basiliximab) in% | 67.4/32.6 |
| Follow-up in years, average | 1.9 |
SD: standard deviation; DM: diabetes mellitus; PD: peritoneal dialysis; CKD: chronic kidney disease; HD: hemodialysis; HLA: human leukocyte antigen; IQR: interquartile interval.
The initial protocol limited the donor age to 65 years, but age limit was gradually extended. A 24.1% of donors were older than 65 years and 45.9% fulfilled the criteria of donors with expanded criteria (Age>60 years or >50 years with 2 of the 3 risk factors:high blood pressure, cardiovascular death or Serum Cr>1.5mg/dl. Regarding the extraction–preservation process, patients underwent a rapid laparotomy with static cold preservation in 61% of cases;cannulation antemortem with a triple lumen catheter and cold perfusion was performed in 16% and, the rest had normothermic perfusion with extracorporeal oxygenation. Extraction of only the kidneys was performed in 64.7% of the cases and multiorganic extraction in the rest. Throughout the time, the tendency has been toward an increase in multiorganic extractions which was almost testimonial during the first year; and, there was progressive increase in the use of machine perfusion models, an aspect that was also included in the report of activities of the ONT.10
The present analysis is focused mainly on clinical outcomes and it is not evaluating the process of donation which concerns to the transpant coordination. Therefore, we do not have information on the percent of family refusals, potential non-effective donors, cost-effectiveness in obtaining other organs or precise data of the process prior to extraction: duration of the agony period, functional ischemia, evolution of the blood pressure or post-extubation oxygen saturation. The ONT annual reports provides detailed information in this regard.10
In total, 670 kidneys were obtained from 335 donors; Of the total kidney grafts removed, 38 were transferred and transplanted in other centers, 25 were transferred but could not be transplanted (the cause is unknown) and 41 were lost to follow-up. At the end, there were 566 kidneys implanted in the transplant hospitals of the GEODAS group (Fig. 1).
Description of the recipients and the transplant processThere were 566 recipients, mostly males (68.4%). mean age 56.5 years (SD 12.0, range 19–83), and 24.1% were older than 65 years. Recipients baseline characteristics are shown in Table 1. For the majority (91.9%) it was the first RT, in a 7.3% it was the second and in a 0.9% was the 3rd or even the 4th transplant. Most recipients were of low immunological risk, none had antibodies greater than 90% and with low HLA compatibility.
The immunosuppression treatment included induction therapy in almost all cases (99.7%), which was Thymoglobulin® (Sanofi, France) in 67.4% and Simulect® (Novartis, Switzerland) in 32.6%. Triple maintenance therapy included prednisone, mycophenolate mofetil/mycophenolic acid and tacrolimus in 91.3% of patients and prednisone, mTOR inhibitor and tacrolimus in 6.5%, adjusting to the patient immunological risk profile according to the local protocol.
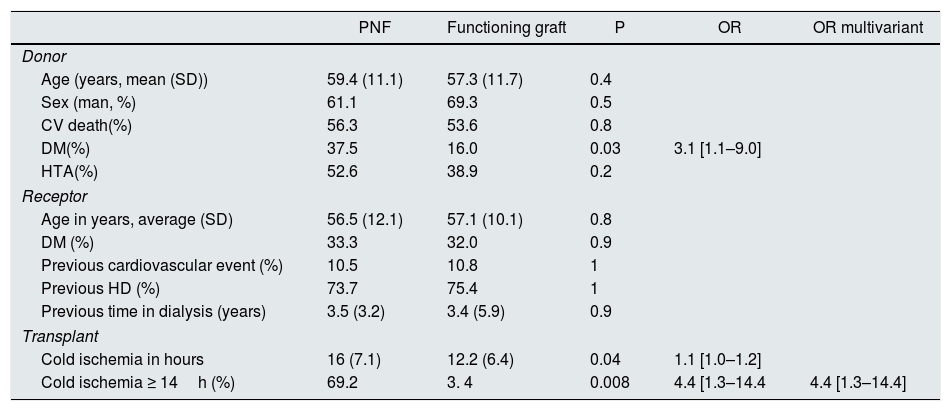
With respect to the graft function, 19 patients (3.4%) never had a functioning graft (PNF). The function of the 19 contralateral kidney grafts was: 2 had DGF, 4 did not have follow-up in our group and 13 had no initial complication. The recipients who presented PNF came from old donors, with higher comorbidity and with a longer CIT (16.0 vs. 12.2, p=0.04). In the multivariate model, only the CIT ≥14h was associated with an OR of 4.4 for PNF (Table 2).
Characteristics of the donor and recipient according to the initial function of the graft. Univariate and multivariate analysis by logistic regression of the risk factors associated with PNF.
| PNF | Functioning graft | P | OR | OR multivariant | |
|---|---|---|---|---|---|
| Donor | |||||
| Age (years, mean (SD)) | 59.4 (11.1) | 57.3 (11.7) | 0.4 | ||
| Sex (man, %) | 61.1 | 69.3 | 0.5 | ||
| CV death(%) | 56.3 | 53.6 | 0.8 | ||
| DM(%) | 37.5 | 16.0 | 0.03 | 3.1 [1.1–9.0] | |
| HTA(%) | 52.6 | 38.9 | 0.2 | ||
| Receptor | |||||
| Age in years, average (SD) | 56.5 (12.1) | 57.1 (10.1) | 0.8 | ||
| DM (%) | 33.3 | 32.0 | 0.9 | ||
| Previous cardiovascular event (%) | 10.5 | 10.8 | 1 | ||
| Previous HD (%) | 73.7 | 75.4 | 1 | ||
| Previous time in dialysis (years) | 3.5 (3.2) | 3.4 (5.9) | 0.9 | ||
| Transplant | |||||
| Cold ischemia in hours | 16 (7.1) | 12.2 (6.4) | 0.04 | 1.1 [1.0–1.2] | |
| Cold ischemia ≥ 14h (%) | 69.2 | 3. 4 | 0.008 | 4.4 [1.3–14.4 | 4.4 [1.3–14.4] |
SD: standard deviation; DM: diabetes mellitus; HTN: hypertension; OR: odds ratio; PNF: primary graft failure.
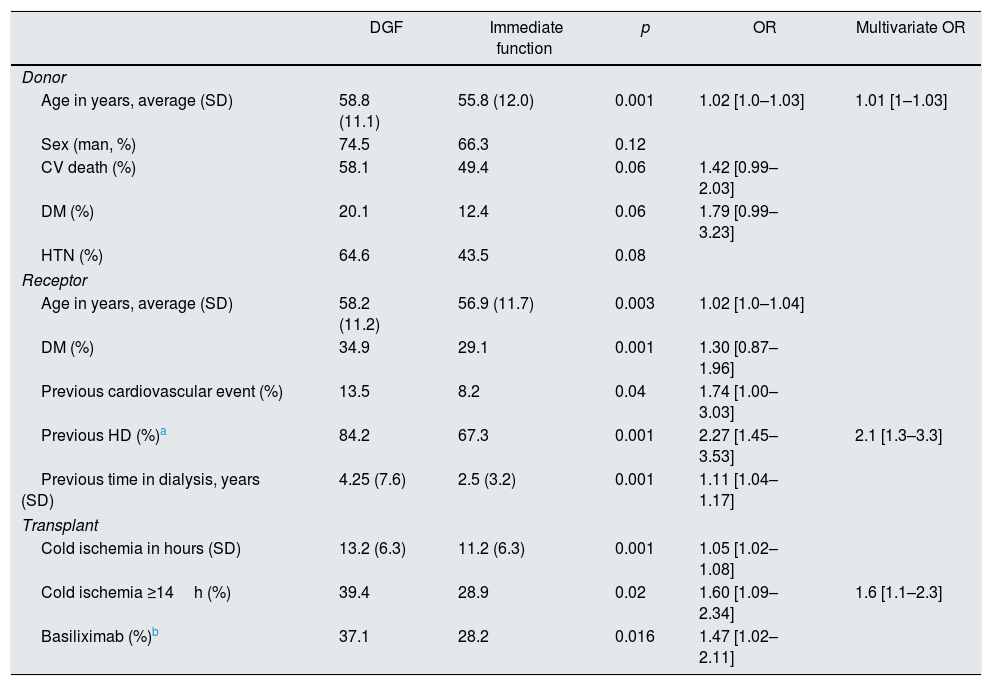
A 48.8% of the recipients developed DGF, with a mean of 9.5 days in hemodialysis treatment (range: 1–94 days, IQR5–15). The patients who presented DGF were older, with more comorbidities, had been more time on dialysis and received organs from older and comorbid donors, with longer CIT. The use of basiliximab was associated with more frequent DGF, but this variable had no influence in the multivariate model. The factors associated to DGF in the multivariate analysis were: CIT ≥14h (OR 1.6 [1.1–2.3]), the type of previous renal replacement therapy with hemodialysis (vs. peritoneal dialysis, OR 2.1 [1.3–3.3]) and age of the donor (per year, OR 1.01 [1–1.03]). The rest of the variables did not show a significant difference (Table 3).
Characteristics of the donor and recipient according to the initial function of the graft. Univariate and multivariate analysis by logistic regression of the risk factors associated with delayed graft function.
| DGF | Immediate function | p | OR | Multivariate OR | |
|---|---|---|---|---|---|
| Donor | |||||
| Age in years, average (SD) | 58.8 (11.1) | 55.8 (12.0) | 0.001 | 1.02 [1.0–1.03] | 1.01 [1–1.03] |
| Sex (man, %) | 74.5 | 66.3 | 0.12 | ||
| CV death (%) | 58.1 | 49.4 | 0.06 | 1.42 [0.99–2.03] | |
| DM (%) | 20.1 | 12.4 | 0.06 | 1.79 [0.99–3.23] | |
| HTN (%) | 64.6 | 43.5 | 0.08 | ||
| Receptor | |||||
| Age in years, average (SD) | 58.2 (11.2) | 56.9 (11.7) | 0.003 | 1.02 [1.0–1.04] | |
| DM (%) | 34.9 | 29.1 | 0.001 | 1.30 [0.87–1.96] | |
| Previous cardiovascular event (%) | 13.5 | 8.2 | 0.04 | 1.74 [1.00–3.03] | |
| Previous HD (%)a | 84.2 | 67.3 | 0.001 | 2.27 [1.45–3.53] | 2.1 [1.3–3.3] |
| Previous time in dialysis, years (SD) | 4.25 (7.6) | 2.5 (3.2) | 0.001 | 1.11 [1.04–1.17] | |
| Transplant | |||||
| Cold ischemia in hours (SD) | 13.2 (6.3) | 11.2 (6.3) | 0.001 | 1.05 [1.02–1.08] | |
| Cold ischemia ≥14h (%) | 39.4 | 28.9 | 0.02 | 1.60 [1.09–2.34] | 1.6 [1.1–2.3] |
| Basiliximab (%)b | 37.1 | 28.2 | 0.016 | 1.47 [1.02–2.11] | |
SD: standard deviation; DGF: delayed graft function; DM: diabetes mellitus; DP: peritoneal dialysis; HD: hemodialysis; HTN: hypertension; OR: odds ratio.
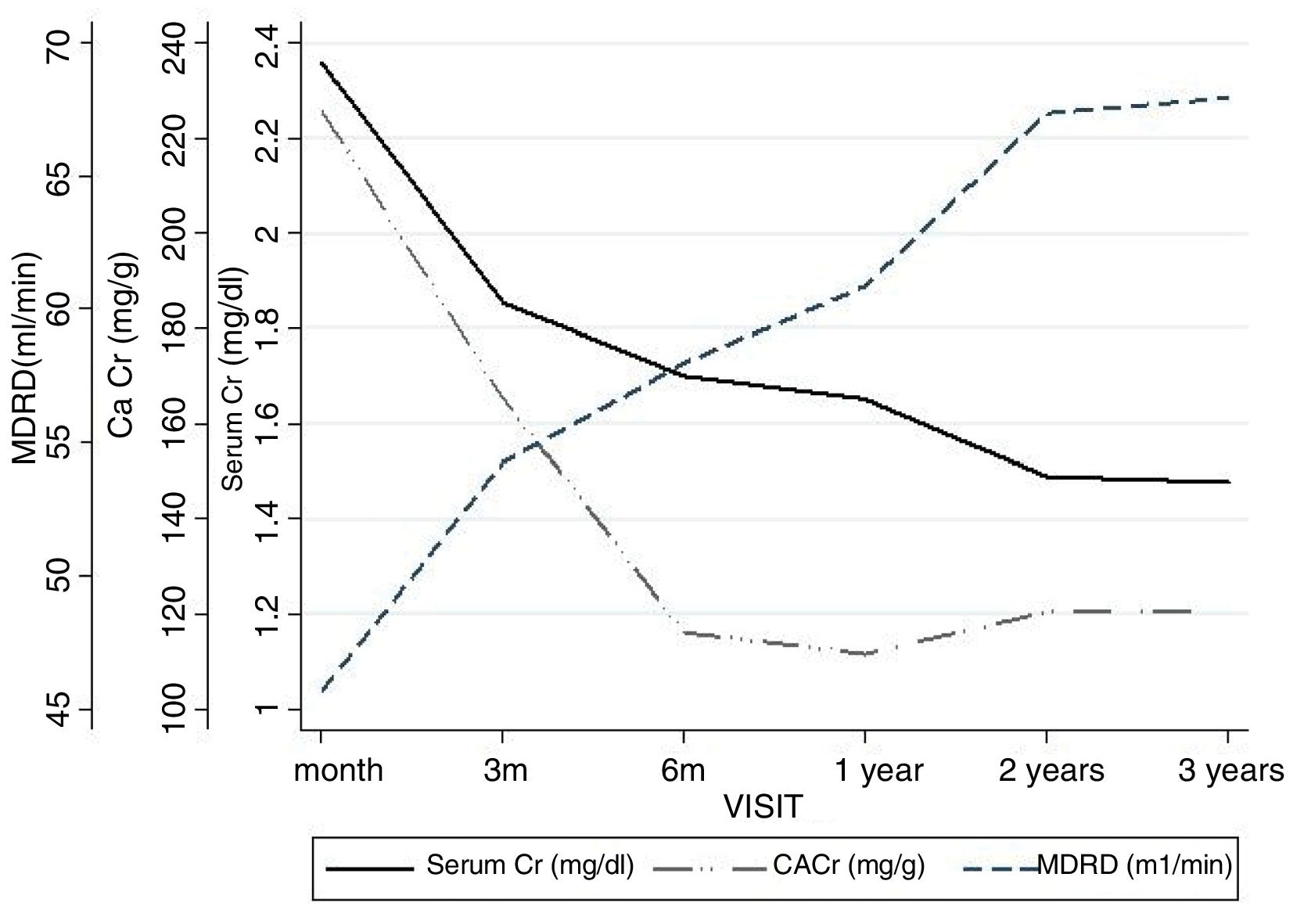
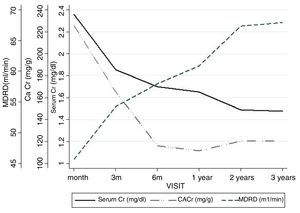
The renal function estimated by MDRD-4 at one year was 60.9ml (SD 24.1). The evolution of serum Cr, estimated glomerular filtration rate and proteinuria are shown in Fig. 2.
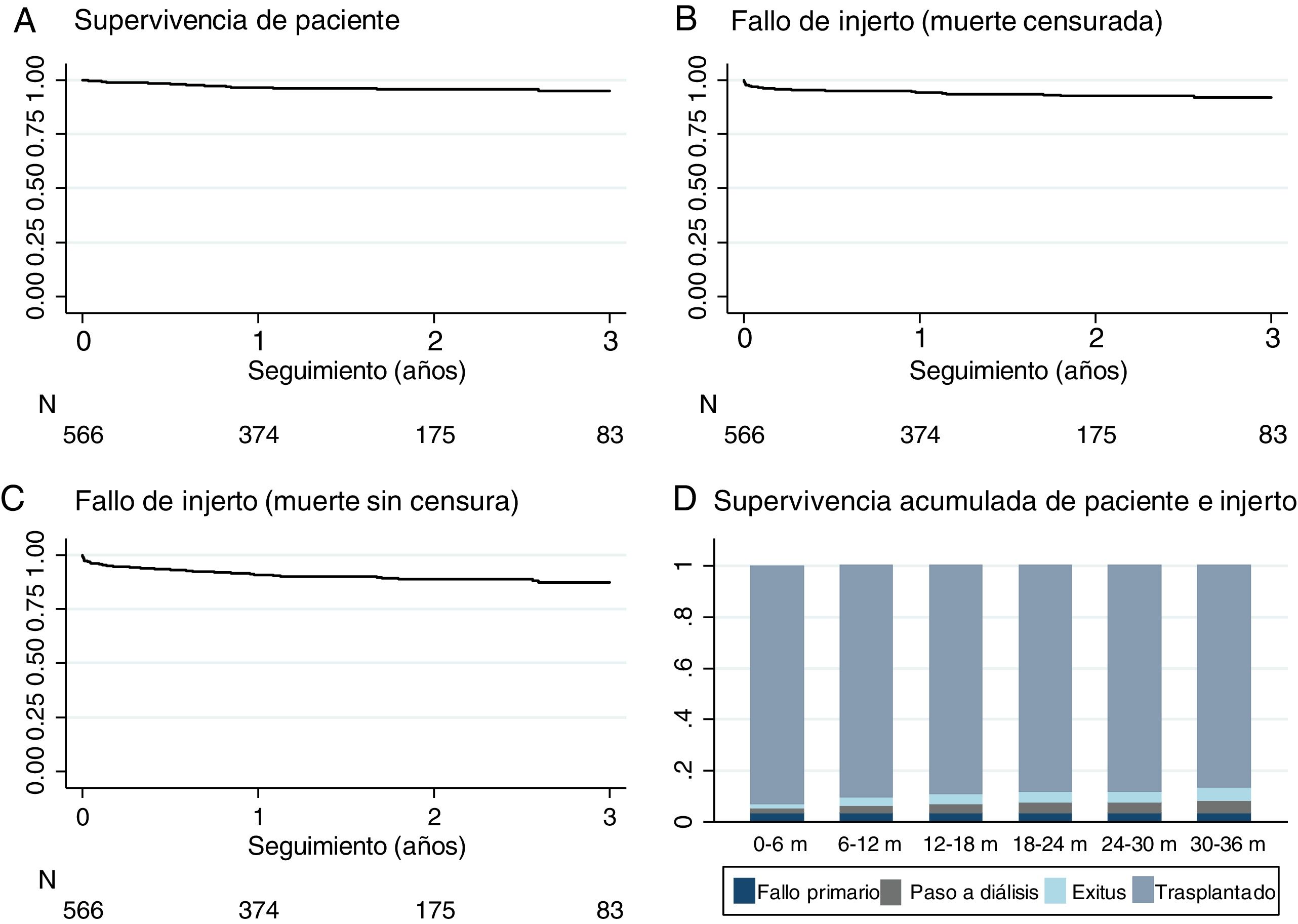
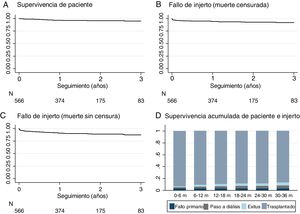
The mean follow-up was 1.9 years, with a range between 3 and 60 months. At the end of the study, 21 patients (3.7%) had died with a functioning graft. The causes of death were: cardiovascular (n=10), infection (n=4), tumor (n=2) others (n=5). A 6.7% returned to dialysis (6% to hemodialysis and 0.7% to peritoneal dialysis) due to PNF or graft failure during the follow-up. Only 0.4% were lost in the follow-up (Fig. 1). Patient survival, estimated by Kaplan–Meier curves, was 96.6% after one year and 95% at 2 years (Fig. 3A). The survival rate of the renal graft censored by death was 97.6% the first year and 95.1% at 2 years, and it was reduced to 94.2% and 91.7%, respectively, if the cases of PNF were included (Fig. 3B). Only 3.4% (n=19) of the grafts that functioned initially presented a definitive failure during the follow-up and required reinitiation of dialysis.
DiscussionThe present work is the first analysis of a large sample of RT from cDCD donation collected from many centers throughout the Spanish territory that allows to evaluate the clinical results. Therefore, it serves as a reference for comparison with other procedures and for the promotion of this donation model. The good initial preliminary results reported by some centers are confirmed with this present analysis.7,8
The cases of RT using cDCD have increase from only less than 2% of all donors in 2012 to 26% in 2017.10 With this strategy, the figures of 46.9 donors pmp and 12 cDCD pmp places us, Spain, at the top of the international ranking.10
The incidence of PNF and DGF described in this report is within the range reported by other publications from countries with more experience in the field. The most relevant reference models for this type of RT are Europeans, particularly from the United Kingdom11 and the Netherlands5 and the furthest model from the United States of America.12 A recent meta-analysis compares the results of 3014 RT performed with organs derived from cDCD with those of more than 80,000 DBD and establishes a risk of DGF more than double (OR 2.74 [2.04–3.68].13 Comparisons of results obtained in different countries requires a review of the different protocols, management models, donation and immunosuppression protocol and the profiles of donors and recipients .14 In our country, the use of this type of cDCD donors has been generalized, so that by the end of 2016, 72 centers had cDCD program. However, the reference models in other countries tend to concentrate the experience in selected centers. United States of America publications refer to a type of donor that is 7 years younger than our donors and a percentage of non-acceptance of the organ of 50% for donors over 65 years. The 13-year experience recently published in the United Kingdom presents an average age for the donor similar to ours11 but in our case the range is wider and includes older donors, up to 83 years, with Cr values above 2mg/dl.
Initially the limit for the cDCD donation was: age <65 years, with normal renal function and low comorbidity.6 However, we have seen a progressive widening of the selection criteria once it was learned that one out of every four donors was over 65 years of age and almost half meet the classic requirements that define a donor with expanded criteria. The results of PNF and DGF did not change with the years, so we assume that we had improve from our experience and that is compensated by the liberalization of the selection criteria and the acceptance of the suboptimal donor.10
Nevertheless, analysis of regional registries show that the mortality risk being maintained in waiting list is greater than receiving an organ from a donor with expanded criteria, for example, over 65 or even 75 years.16,17
The pathophysiological process of ischemia-reperfusion injury is different in cDCD and DBD18 which explains a higher incidence of DGF. The agony process prior to cardiac arrest entails a time of warm ischemia that cannot be avoided, and contributes to the organ damage in DBD.18 In the case of uncontrolled DCD, the first period of warm ischemia is much more prolonged, which explains that the DGF is almost the norm.10 In our study, DGF occurs in one out of 2 cases, despite the systematic use of induction therapy and delayed introduction of tacrolimus as a strategy for renal protection in some centers. We have identified the CIT as the most relevant modifiable risk factor and it is established a limit of hours that predicts a significant increase in risk. The annual reports of the ONT shows that there is an increase in the use of machine perfusion of donor with ECMO reaching 30% in 2016.10 The nonuniform definition of DGF event limits the comparison between studies. In our case, DGF is defined as a need for hemodialysis in the first week post-TR, which could justify the higher incidence observed in our series, since cases of DGF can be included with a single session of hemodialysis due to difficult management of water overload. Therefore, it is possible that the lower incidence of DGF in patients that had not received dialysis prior to RT (anticipated RT) or coming from peritoneal dialysis is partly due to a greater residual renal function and less immediate access to the urgent hemodialysis session. This finding is common in other studies.19,20
Although PNF cases are mainly due to problems with organ conservation or surgical procedure, our study shows that CIT is the most important modifiable risk factor. Strategies to reduce early events such as PNF and DGF should focus on improving organ conservation systems and reducing CIT. This is the reason why some authors recommend minimizing this time by sending one kidney to another nearby hospital to achieve in both transplants a CIT less than 12h.21
In a local study already published5 we did not find that DGF had an impact on the posterior survival of the organ. A recent study of great statistical power compares pairs of recipients from the same donor in which one recipient develops DGF and the other had immediate function. This analysis on almost 30,000 RT eliminates the confounding factors of the donor. The risk of graft failure during the first year increases in those with DGF HR 3.75 [3.31–4.24] and even more in those with some acute rejection added to DGF (HR 8.16 [4.72–14.10]). This effect loses strength during the following years, with a risk of 1.16 (1.02–1.31) and 1.19 (0.59–2.23). In addition, DGF carries difficulties with both diagnosis and management; there are uncertainty with the diagnostic, requiring early biopsies and adjustments of immunosuppression, prolonged admission and the cost per process increases.19
Renal function at the first year was close to 60ml/min, so we could be expected good medium and long term survival. Our study does not allow a comparison with the results of the DBD donation, but a recent analysis of the Andalusian regional registry shows that renal function achieved after one year is better in transplants from DBD donors than from DCD (Crs 1.79±0.90 vs. 1.46±0.50mg/dl; p<0.001).22
Although we have collected cases with a follow-up of up to 5 years, our average follow up only allows us to analyze survival at the second year. Survival of the patient and organ censored by death with a functioning graft are similar to the records for DBD.10 The analysis of the risk factors for survival is outside the scope of this first study; such analysis we will performed once solid conclusions can be obtained based on a longer follow-up.
The main limitations of the present study are a mean follow-up of only 2 years and also the absence of a common protocol for the extraction and preservation of the organ, as well as for the immunosuppression treatment regimen. However, we have included more than one third of all RT with organs from cDCD10 and the sample size is sufficient to adequately represent the general situation in Spain. The direct involvement of clinicians in the design of the database and in the collection of information is a guarantee of the quality of the information.
Some authors warned about the risk that the cDCD donation would anticipate to the DBD, so an increment in organ donation may not be achieved.11,20 Although this analysis is outside the framework of the GEODAS-3 study, we can use the reference of the ONT records to confirm that the cDCD model has supposed a net increase of donors without reduction of the DBD donation rates,10 something that confirms the model of the United Kingdom after 13 years.11 The present work adequately represents the reality of RT with the cDCD donation model and contributes to its promotion and implantation in the rest of the transplant centers in our country.
ConclusionsThe cDCD programs has been extended because they present an organizational complexity that is quite accessible and allows an increase in rate of donation without compromising the DBD. The learning curve seems to be accomplished, and our results are similar to those reported by countries with a long tradition. The CIT is the most relevant modifiable risk factor. Pending an analysis of organ and patient survival in the longer term, we have our own and current results that support the development of this joint strategy.
FinancingThis project is sponsored by the SENRA Group of the SEN, the RedinRen 16/009/009 RETYC ISCIII, the Renal Iñigo Álvarez de Toledo Foundation and a collaborative grant from Novartis, Astellas and Sanofi through the Puerta de Hierro Health Research Institute-Segovia Arana.
Conflicts of interestThe authors declare that they have no conflict of interest.
List of centers that make up the GEODAS group:
Puerta de Hierro University Hospital (Madrid), Hospital del Mar (Barcelona), Germán Trías and Pujol University Hospital, Carlos Haya University Hospital (Málaga), La Fe University Hospital (Valencia), Reina Sofía University Hospital (Córdoba), University Hospital Puerta del Mar (Cádiz), Gregorio Marañón University General Hospital, (Madrid), Cruces University Hospital (Bilbao), University Hospital of Vall d’Hebron (Barcelona), University Hospital Clinic of Barcelona, Bellvitge University Hospital (Barcelona), Marqués University Hospital of Valdecilla (Santander), University Hospital of Albacete, Miguel Servet University Hospital (Zaragoza), Puigvert Foundation (Barcelona), University Clinic of Navarra, San Carlos Clinical University Hospital (Madrid), University Hospital Complex A Coruña, University Hospital of La Paz (Madrid), Dr. Peset University Hospital (Valencia), Jiménez Díaz Foundation (Madrid).
Please cite this article as: Portolés JM, Pérez-Sáez MJ, López-Sánchez P, Lafuente-Covarrubias O, Juega J, Hernández D, et al. Trasplante renal con órganos procedentes de donación tras parada circulatoria controlada: resultados del estudio multicéntrico GEODAS-3. Nefrologia. 2019;39:151–159.