Since the start of vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), various groups of researchers have found a weak immune response in patients with solid organ transplants.1,2 This finding and the fact that some transplant patients have suffered from COVID-19 after being fully vaccinated with two doses, has led to the recommendation, in some countries, of a third dose of vaccine in these patients.3,4
We prospectively studied the humoural response to the mRNA-1273 (Moderna) vaccine in 73 kidney transplant recipients by quantitatively determining anti-Spike IgG antibodies to SARS-CoV-2, immediately before the second dose and eight weeks after vaccine administration, analysed by microparticle chemiluminescence (Abbott Alinity system [ref. value + >50 AU/ml]). The results were compared with the response to the same mRNA-1273 vaccine administered to 30 patients on haemodialysis (HD), 12 on peritoneal dialysis (PD), 21 pre-dialysis (pre-D) and 47 controls, who were healthy hospital workers.
As this was a prospective study, it was approved by the Independent Ethics Committee for Clinical Trials with medicines (IECm) of the institution, Gerencia de Asistencia Sanitaria de Ávila [Ávila Healthcare Management].
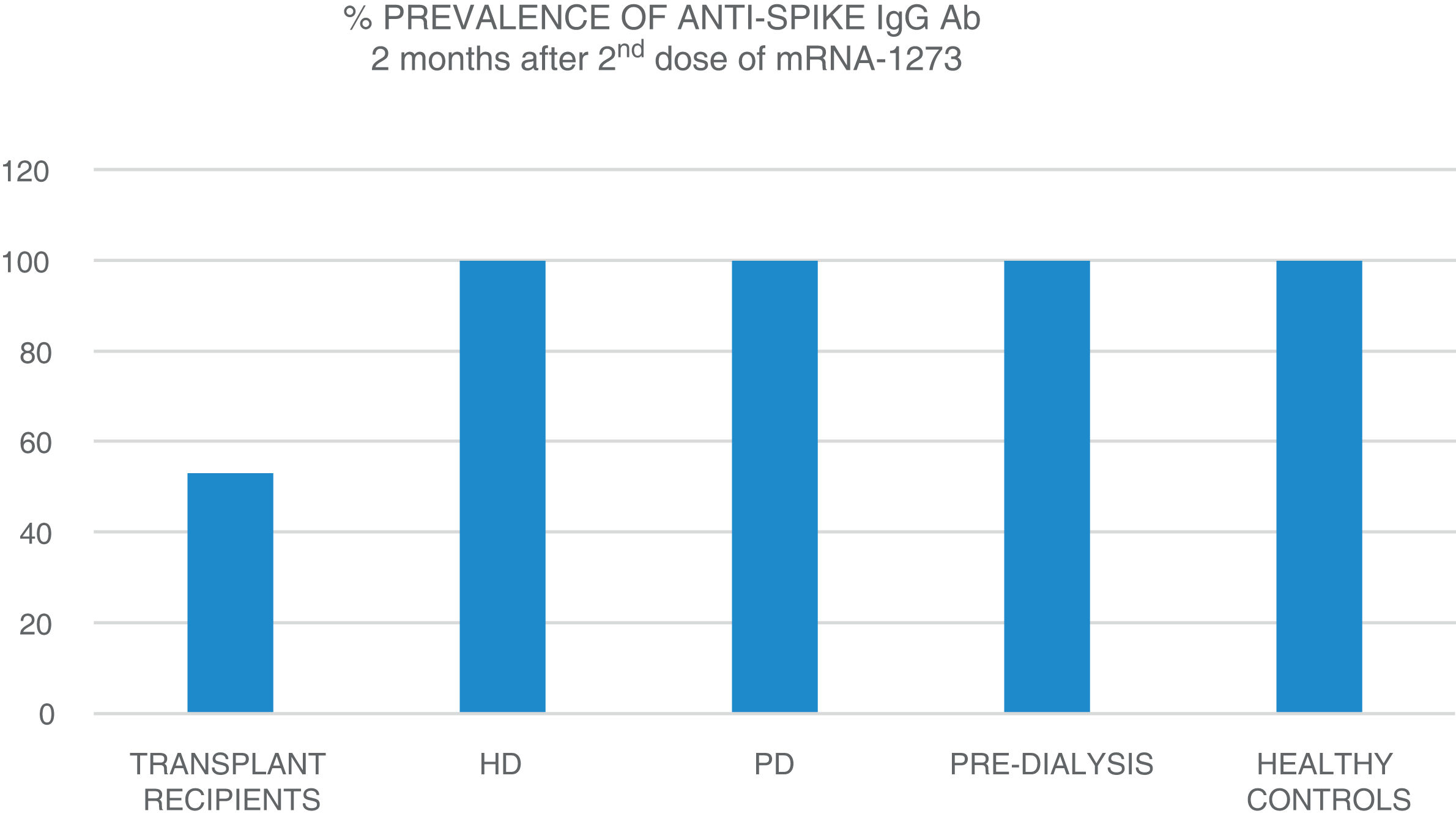
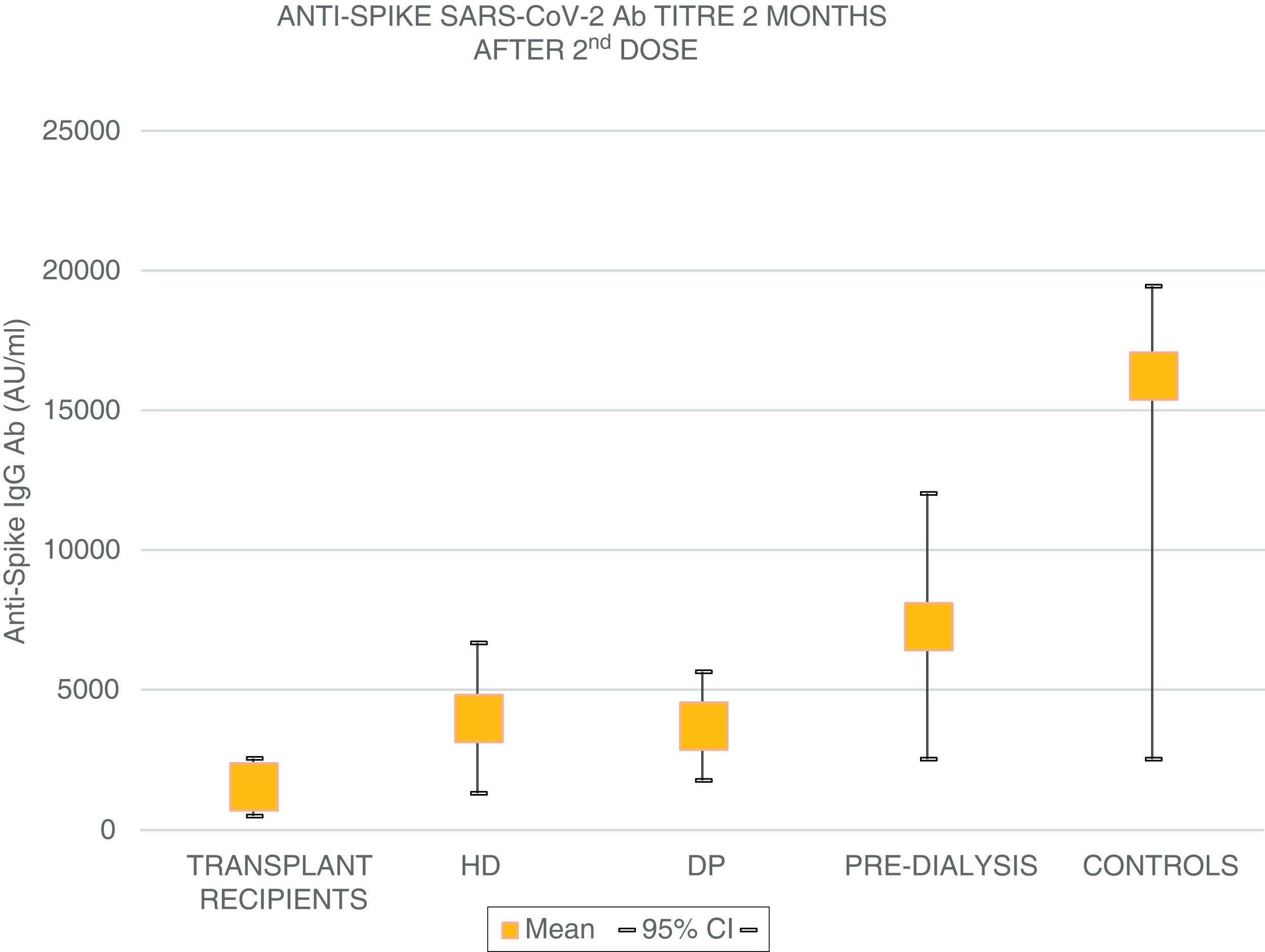
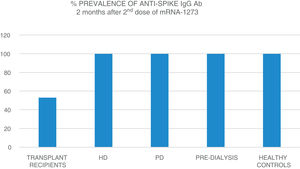
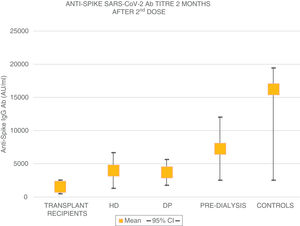
The mean age of the transplant recipients was 60.12 ± 10.4 and the time since transplantation was 123–11,888 days; 56.1% were male. Four patients who had previous SARS-CoV-2 infection and three patients who had been in close contact and had a high suspicion of infection had very high titres (40,374.6 ± 55,211.5 AU/ml) and were excluded from the analysis. The response to the first dose of the vaccine, defined by IgG levels >50 AU/ml, was only found in 16.4% (11 patients) with a mean anti-Spike IgG antibody (Ab) titre of 270.8 ± 322.0 (median: 172.9; r = 52.6–19,650.3); 79.8% had levels below 50 AU/ml, and 21 (28.7%) were anergic (0–<1 AU/ml). Eight patients on HD, one on PD and two pre-D had already had clinical SARS-CoV-2 infection and all developed a strong anti-Spike Ab response to the first dose of vaccine (patients on HD: 42,181 ± 22,798; PD: 35,418.4 AU/ml; pre-D: 36,934.3 ± 35,026.7). Of the rest, 90.9% of patients on HD, 72.7% of those on PD and 78.9% of the pre-D developed an Ab response >50 AU/ml, with mean figures of 377.5 ± 350.4 AU/ml (median: 314.9) in HD; 1176.5 ± 1823.8 (median: 646.7) in PD and 1158.3 ± 1431.1 (median: 683.6) in pre-D, with significant differences compared to the transplant recipients (p = 0.004). The transplant recipients with IgG anti-Spike <50 AU/ml were older, 63.7 ± 9 vs 58.5 ± 10 (p = 0.1), with more lymphopenia 1568 ± 731 vs 2060 ± 680 (p = 0.05); and there was a correlation between IgG anti-Spike and lymphocytes: R = 0.32 (p = 0.007); a certain negative correlation was also detected with tacrolimus, R = −0.24, and prednisone, R = −0.14, although not statistically significant (p = 0.1). Eight weeks after the second dose of mRNA-1273, another antibody determination was performed; 35 transplant recipients already had levels >50 AU/ml, (53.03% vs 100% of patients on HD, PD, pre-D and healthy controls) (Fig. 1). The anti-Spike Ab titre was also lower than the rest of the groups: mean 1544.11 ± 4279.58 AU/ml (95% CI: 2576.58–511.6) vs 4000.3 ± 5567.2 (95% CI: 6683.62–1317.03) on HD; 3709.3 ± 2889.6 (95% CI: 5650.61–1768.03) on PD; 7288.4 ± 7849.1 (95% CI: 12,031.6–2545.2) in pre-D; and significantly lower than those of healthy controls: mean 16,226.3 ± 11,319.8 (95% CI: 19,462.5–2545.2) (Fig. 2). There was no evidence of any serious events after the first or second doses, or any rejection phenomenon or variation in serum creatinine. In the follow-up period, none of the patients or controls developed COVID-19 infection, despite having a low antibody titre, perhaps related to a certain degree of cellular immunity.5
This study shows that, in transplant recipients, the immunisation achieved with the second dose of mRNA-1273 (Moderna) vaccine improves considerably, but without reaching a prevalence of at least 70% in this population, with anti-Spike anti-IgG Ab titres significantly lower than the controls and the rest of the groups of patients with advanced chronic kidney disease (ACKD). As a large proportion of transplant recipients remain at risk of COVID-19, another third dose of vaccine should be provided as soon as possible, as is already being done in other countries.6