To the Editor,
Parkinson’s disease (PD) is a common neurodegenerative disease that can be caused by mitochondrial dysfunction, oxidative stress, apoptosis or inflammation.1 Between 50% and 80% of PD patients show intolerance to glucose, which can be exacerbated by levodopa treatment.2 We describe the case of a patient with PD and poorly controlled diabetes mellitus, who was initially treated with anti-diabetic drugs and later required insulin therapy and who came for consultation with a nephrotic syndrome (NS).
The patient was a 74-year-old man with a 9-year history of diabetes mellitus (initially treated with anti-diabetic drugs and for the last 3 years with insulin); diagnosed with infarctional ischaemic heart disease and post-infarction angina, he had undergone double coronary bypass surgery. Previous episodes of deep vein thrombosis and pulmonary thromboembolism, and hypercoagulability had been confirmed (heterozygotic mutation of homocysteine gene). For 10 years he had had PD, which was being treated with carbidopa/entacapone/levodopa, ropinirole and rasagiline. Other medical conditions included prostate adenoma, hiatus hernia and chronic renal failure with previous plasma creatinine levels of 1.4-1.5mg/dl.
The patient was referred to the emergency department by his GP, owing to symptoms of anasarca. In the days prior to his visit to the emergency department he had noticed a decrease in the frequency of diuresis accompanied by weight gain. He did no report blood-stained or dark-coloured urine. A week before he had developed very itchy petechiae on his arm and the back of his hands.
The physical examination revealed that the patient’s general condition was good, and he was conscious and orientated. There was slight jugular vein ingurgitation and his blood pressure was 150/78mm Hg. Body temperature was normal. As far as the rest of the examination is concerned, notable symptoms included pitting oedema of the lower limbs and signs of venous insufficiency.
In the complementary tests, the blood analysis showed: haematocrit: 44%, leukocyte: 7060, platelets: 152 000; pH: 7.32, bicarbonate: 28mEq/l, glucose: 241mg/dl, creatinine 2.2mg/dl and calcium 7.7mg/dl. The rest of the on-the-spot analysis was normal.
In the routine blood analysis the findings were as follows: uric acid: 11.8mg/dl, cholesterol: 297mg/dl, triglycerides: 141mg/dl, albumin: 1.9g/dl, total protein: 5.2g/dl, LDH: 629U/l, glycosylated haemoglobin: 8.5%. Immunological analysis: C-reactive protein 1.9mg/dl; rheumatoid factor, ASLO, ANCA, antinuclear antibodies, anti-Ro, anti-La, anti-Sm and anti-RNP antibodies were within normal limits. Tumour markers, including ACE, CA19-9, AFP and PSA were acceptable. Blood electrophoresis: hypoproteinaemia, reduced albumin levels, raised alpha-2 and beta globulins with a polyclonal increase in gammaglobulins. Thyroid hormones were normal. Serological tests for the hepatitis C and HIV virus were negative. HBsAg positive, anti-HBc and anti-HBs negative; hepatitis B virus DNA less than 2000 copies/ml. Herpes virus 1-2 IgG positive.
The urine analysis on admission showed proteins +++, blood ++ and the presence of casts (cylindruria). Protein quantification in 24-hour urine was 13g/24 h.
Chest X-ray: enlarged heart with no signs of acute heart failure. Electrocardiogram: sinus bradyarrhythmia at 50bpm. A Doppler ultrasound scan showed no pathological findings.
Given the patient’s history of poorly controlled diabetes mellitus and his admission owing to recent fluid retention, it was decided that a renal biopsy should be performed. Our findings were as follows: six glomeruli, two of which were completely sclerotic. In two of the other four glomeruli, focal, nodular lesions of the glomerular tuft (Kimmelstiel-Wilson nodules) were identified. The result of the immunofluorescence assay was negative. Moderate interstitial fibrosis associated with tubular atrophy and chronic inflammatory infiltrate was observed. The vascular component presented no lesions. The definitive diagnosis was nodular glomerulosclerosis with a morphological substrate of diabetic nephropathy (DN).
With this diagnosis, the initially established treatment, which consisted of diuretics, irbesartan, atenolol, statins and oral anticoagulants, was maintained and the patient was discharged.
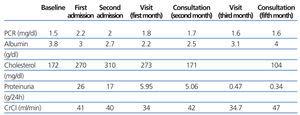
Twelve days later the patient was re-admitted for fluid retention, and he responded favourably to diuretic treatment. Subsequent outpatient follow-up showed the analytical changes depicted in Table 1 and the patient has not presented new episodes of fluid retention.
DN is a common complication of diabetes and is currently an important public health problem, as diabetic renal disease is the main cause of terminal chronic kidney disease in Western countries.3 Diabetic patients with a history of DN who develop slow-onset proteinuria, are not usually subjected to a biopsy, on the assumption of the presence of DN. However, non-diabetic glomerular disease may also develop in diabetic patients, which is why a renal biopsy may be indicated.4 In our case, the patient had longstanding diabetes mellitus, which was poorly controlled metabolically. We are unaware whether he had proteinuria prior to his first admission, although the onset of anasarca and fluid retention was sudden, so we decided to perform a renal biopsy and the diagnosis was DN.
In the medical literature cases of NS due to minimal change disease have been reported in diabetic patients.5,6 In the case described by Donaire et al, the suspicion of a cause other than diabetes was founded on the short history of diabetes, the absence of retinopathy and the fact that a previous check-up proved negative for proteinuria.5 Although in our case the established diagnosis was DN, the sudden onset of symptoms with severe proteinuria which led to fluid retention on more than one occasion and subsequently spontaneous remission, and then a proteinuria of less than 0.5g/24 h during follow-up, suggested the possibility that the patient might have a comorbid minimal change nephropathy. This might have gone unnoticed during the histological analysis when an underlying DN substrate was found and electron microscopy test was not performed. The patient might have had an unrelated infectious process prior to his first admission. In fact, he had developed cutaneous lesions on his upper limbs. This process could have been triggered by an immune mechanism, leading to an increase in glomerular permeability and, subsequently, severe NS with spontaneous remission some months later.
To conclude, we described a case of NS with clinical symptoms indicating a minimal change aetiology, which could have gone unnoticed in the renal biopsy because we found a DN histological substrate associated to the base pathology (long-term diabetes mellitus).
Table 1. Follow-up of laboratory results