In our country, there is a long tradition of designing and maintaining records of the most prevalent kidney diseases such as glomerulonephritis (GN) and pathologies diagnosed by kidney biopsy. The studies on the epidemiology of biopsy kidney diseases in Spain, especially glomerular pathologies, was started in 1986 when the decrease in the incidence of membranoproliferative GN (MPGN) was independently described in two hospitals in Madrid.1,2 At that time, a publication in the journal Nefrología insisted on the need to join efforts to create study groups with the purpose of obtaining consistent and reliable multicenter data.3 Shortly after, in 1987, the first reports were published, both in the child population (1364 cases)4 and in the adult population (8545 cases),5,6 including kidney biopsies performed between 1970 and 1986. These publications, made by the SEN Study Group, mark the beginning of continued and productive work at the national level, a fact that was favorably commented on and encouraged by the then Director of the Nephrology Journal.7 A few years later, in 1990, a multicenter study was published, including 2123 kidney biopsies, carried out in 1987 and 1988, which consolidated the so-called "Spanish Registry of Glomerulonephritis (REGN)".8 Based on this registry there were several publications in the 90 s, reporting the results of kidney biopsies nationwide, with a significant percentage of participation by the Nephrology Services or Units of the entire country.9–15 In 1994, there were two new features introduced in data collection: a) filling individualized information of each patient to facilitate statistical processing and b) inclusion of non-glomerular pathology. With the information collected with a record for each renal biopsy, the first publication was made in Nephrology Dialysis and Transplantation in 2002,16 which had a high impact. In summary, 23 manuscripts have been published4,8–27: 12 in Nefrologia4,8,10–15,25 and 11 in other international journals16,26,27 (el numero de publicaciones no se corresponde con el numero de referencias). The reliability of the registry was demonstrated by the publication of detailed information about the incidence and prevalence of the different renal histological forms,6,8,10–13,15,16,25 as well as its correlations with the clinical expression,18 both in the child4 and in the elderly.14,20 In addition, certain clinical syndromes such as: acute renal failure,19 hematuria26 and nephrotic syndrome9 have been studied in depth. Various renal entities have been analyzed, such as IgA nephropathy,27 extracapillary GN,24 membranoproliferative GN,4,5,17 lupus nephritis,21 amyloidosis23 and acute tubulointerstitial nephritis.22 Since 1988 these data have been presented in the session of Registries at the annual congresses of the SEN, downloadable from the SEN website.28 To facilitate participation, for about 15 years, data has been sent electronically through the SEN website. As a last novelty, there has been stablished a link to send biopsy material to the SEN Biobank (https://www.senefro.org/modules.php?name=webstructure&idwebstructure=30) with the objective to collaborate with applied research using techniques of molecular biology and precision medicine.
With these information, it is confirmed that REGN is one of the largest and most reliable registries worldwide, since the mentioned manuscripts are included among the references of the research dealing with the epidemiology of the kidney biopsy diseases.
It is well known that kidney disease registries provide key information to be applied in clinical practice since it provides knowledge of the most frequent pathologies with real data on their incidence and prevalence,29 it facilitates the implementation of prevention and treatment protocols, as well as serving as the basis for multicenter clinical studies and trials. One of the fundamental points in of a registry is collaboration nationwide, with a form of data entry previously agreed,31 and a uniform policy of indication of renal biopsy32,33; the information obtained helps to answer unresolved questions about prevention and treatment of kidney diseases.34 As discussed in a previous publication, these were the reasons for the generation and development of the REGN.35 Another reason for having a registry of biopsied kidney diseases is that many pathologies, especially glomerular diseases, can only be diagnosed with certainty by doing a renal biopsy.30 A good example of this is the value of renal biopsy in acute renal failure36 or the study of glomerular pathology in elderly patients.37–39 Finally, the information provided with the registry may contribute to assess the overload that these diseases have on the health system due to the number of hospitalizations, progression towards advanced renal failure and deaths.40,41
Studies from a single center give relevant but insufficient information. However, a number of studies carried out in a single reference center have been published, such as studies from in Springfield and Minnesota in the US,42,43 Colombia,44 Ireland,45 Helsinki,46 Belgium,47 Germany,48,49 Porto (Portugal),50 Macedonia,51 Serbia,52 Poland,53 India,54 Japan,55 China,56 Korea,57 Thailand,58,59 Egypt,60,61 South Africa,62 South Asia63, Pakistan.64 In addition, local data have been published about certain renal syndromes, such as nephrotic syndrome in Chicago,65 China66 and Pakistan,67 acute kidney failure in Chicago68 and India69 and urinary disorders in Serbia.70 And also about specific entities as referents about the IgA N in Kentucky and in 24 states of the South and Midwest of USA,71,72 Focal Segmental glomerulosclerosis (FSG) in USA73,74 and Brazil,75 primary GN in Sao Paulo (Brazil)76 and UK,77 membranous nephropathy in UK,78 renal vasculitis in Norfolk (UK),79 extracapillary GN-pauci immune in Stockholm80 and Estonia,81 and thin basement membrane in Limburg (Netherlands).82 Finally, some studies have focused on certain age groups such as the child population in China83 and South Asia63 or in patients ≥ 60 years in Chicago,68 ≥ 65 in Ireland,45 Japan84 and Turkey85 and in ≥ 80 years in Japan86 and USA87.
National or multicenter registries are the most reliable to know the epidemiology of biopsied kidney diseases and allow comparisons; in Table 1, are indicated most of these studies, including those made in the UK,88–91 France,92,93 New Caledonia,94 Italy,95–101 India,102 United Arab Emirates,103 Japan,104–118 Denmark,119,129 Singapore,121 Peru,122 Australia,123 Korea,124 China,125 Czech Republic,126,127 Hungary,128 Croatia,129 Uruguay,130 Romania,131,132 Brazil,133–136 Lebanon,137 States States,138 Lithuania,139 Turkey,140 Colombia,141 Norway142–144 and Poland,145 among the most important. IgA Nephropathy is the most frequent nephropathy in Europe and Asia, while in the USA the FSG could be increasing.42,73,74,146
Multicenter publications on biopsied kidney disease.
| Authors | Country | No. biopsies | Comments |
|---|---|---|---|
| Johnston et al.88 | 599 | IgAN data | |
| Johnston et al.90 | UK | 255 | NS data in the elderly |
| Hedger et al.89 | 241 | Renal vasculitis data | |
| McQuarrie et al.91 | 2480 | Incidence variations in Scotland | |
| Simon et al.92 | 942 | IgAN as the most frequent | |
| Simon et al.93 | France | 1742 | IgAN as the most frequent |
| Painter et al.94 | New Caledonia | 275 | IgAN and FGS as the most frequent |
| Stratta et al.95 | 1926 | IgAN as the most frequent | |
| Schena et al.96 | 15,461 | IgAN and MN as the most frequent | |
| Coppo et al.97 | Italy | 432 | IgAN and Henoch – Schönlein purpura nephropathy in children |
| Vendemia et al.98 | 2511 | MN and proliferative extracapillary GN in ≥ 65 years | |
| Gesualdo et al.34 | 14,607 | IgAN and nephroangiosclerosis as the most frequent | |
| Lupo et al.99 | 816 | IgAN as the most frequent in primary GN | |
| Zaza et al.100 | 2680 | IgAN as the most frequent | |
| Zaza et al.101 | 1185 | IgAN, FSG and MN in patients with advanced renal failure | |
| Chugh et al.102 | India | 2947 | High prevalence of nephrotic syndrome: minimal changes, amyloidosis (leprosy and other infections) |
| Yahya et al., 103 | United Arab Emirates | 490 | Predominance of proliferative GN and MN |
| Research Group on Progressive Chronic Renal Disease.149 | |||
| Iseki et al.105 | 1850 | High prevalence of IgAN | |
| Imai et al.106 | Japan | 2832 | Renal survival according to renal biopsy results |
| Sugiyama et al.107 | 281 | Amyloidosis review | |
| Yokoyama et al.108 | Japan | 2400 | IgAN and MN prevalence |
| Yokoyama et al.109 | 2082 | Data from patients ≥ 65−83 years (prevalence of vasculitis ANCA + ) | |
| Kawamura et al 110 | 3073 | Data in the elderly | |
| Sugiyama et al.111 | 79 | MN data | |
| Yokoyama et al.112 | 4016 | MPGN data | |
| Yokoyama et al.113 | 438 | IgAN and MN prevalence | |
| Nakashima et al.114 | 328 | Data on Nephrotic syndrome in patients ≥65 years | |
| Hiromura et al.115 | 47 | Data on drug induced nephropathy | |
| Nishi et al.116 | 331 | Data on IgG-4 associated disease | |
| Komatsu et al.117 | 281 | LN data | |
| Nakaga wa et al.118 | 152 | Amyloidosis data | |
| 593 | Data from Henoch-Schönlein nephritis in ≥ 65 years | ||
| MPGN data | |||
| Heaf et al.119,120 | Denmark | 2380 | High prevalence of proliferative mesangial GN and MC |
| Woo et al.121 | Singapore | 2102 | High prevalence of proliferative mesangial GN and MC. Role of environmental antigens |
| Hurtado et al.122 | Peru | 1263 | Prevalence of LN and MPGN |
| Briganti et al.123 | Australia | 2030 | Prevalence of IgAN and FSG |
| Choi et al.124 | Korea | 4514 | Prevalence of MC and IgAN |
| Li et al.125 | China | 13,519 | Prevalence of IgAN and LN |
| Rychlik et al.126 | Czech Republic | 4004 | Prevalence of IgAN and MC |
| Maixnerova et al.127 | 10,472 | Prevalence of IgAN, MN, LN and MC | |
| Horvatic et al.129 | Croatia | 922 | Prevalence of IgAN, FSG and MN |
| Sipiczki et al.128 | hungr ed | 798 | Prevalence of IgAN and MN |
| Mazzuchi et al.130 | Uruguay | 2058 | Prevalence of FSG and LN GSF and NL prevalence |
| Covic et al.131 | Romania | 635 | Prevalence of MPGN and mesangioproliferative |
| Gusbeth-Tatomir et al.132 | 336 | Prevalence of MPGN and mesangioproliferative | |
| Malafont e et al.133 | 2086 | Prevalence FSG and MN | |
| Polito et al.134 | Brazil | 9617 | Prevalence FSG and MN |
| Costa et al.135 | 1151 | Prevalence FSG and GNMP | |
| Machado et al.136 | Brazil | 582 | Prevalence of FSG, MC and IgAN |
| Karnib et al.137 | Lebanon | 1327 | Prevalence of mesangioproliferative GN and FSG |
| Layton et al 138 | U.S | 217 | Difficulty classifying results of kidney biopsy |
| Brazdziute et al.139 | Lithuania | 5368 | Prevalence IgAN and FSG |
| Fidan et al.140 | Turkey | 3982 | Child population data: prevalence of FSG and Henoch-Schönlein purpura |
| Barrera et al.141 | Colombia | 12,613 | Prevalence of Glomerulosclerosis, IgAN and LN |
| Norby et al.142 | 178 | LN data | |
| Bjorneklett et al.143 | Norway | 81 | Renal Vasculitis ANCA + in the elderly |
| Bjorneklett et al.144 | 357 | Evolution of Renal Vasculitis ANCA + according to gender | |
| Perkowska-Ptasinska et al.145 | Poland | 3934 | Prevalence of IgAN and FSG |
MC: minimal changes; GN: glomerulonephritis; MPGN: membranoproliferative glomerulonephritis, FSG: focal segmental glomerulosclerosis; IgAN: IgA nephropathy; LN: lupus nephropathy; MN: membranous nephropathy; NS: nephrotic syndrome.
The REGN, which has more than 28,000 kidney biopsies in 2018, is only comparable to the registries of Italy34 and Japan118 that have collected, at least until their latest publications, between 15,000 and 26,000 biopsies, respectively.
In the present publication we want to summarize in broad terms the results of our REGN registry, think about the future, examine the current data in 2019, as well as the innovations necessary to maintain it updated and useful.
Present, updated dataDuring the last 26 years (from 1994 to 2019), 27,116 kidney biopsies have been collected (92% first biopsy), with an average of 1042 biopsies per year. A total of 157 Nephrology Units have participated, the list is shown in the Appendix A.
The years of study have been grouped into 5 periods of 5 years each (except the last one that includes 6 years): 1994−1998, 1999−2003, 2004−2008, 2009−2013 and 2014−2019. The ages at the time of the biopsy they have been separated into 4 intervals: < 15 years, 15−65 years, 65−80 years and> 80 years. Histological diagnoses have been grouped into 5 sections: primary GN, secondary GN, tubulointerstitial nephropathy and vascular nephropathies. The rest (hereditary or difficult to classify) have been labeled as"other".
Table 2 indicates the number and percentage of biopsies performed in each time interval according to the age group.
Number and proportion of kidney biopsies by time periods.
| Age (years) | 1994−1998 n (%) * | 1999−2003 n (%) * | 2004−2008 n (%) * | 2009−2013 n (%) * | 2014−2019 n (%) * | Total N (%) * |
|---|---|---|---|---|---|---|
| < 15 | 487 (8.5) | 292 (6.1) | 188 (3.2) | 116 (2.2) | 148 (2.9) | 1231 (4.6%) |
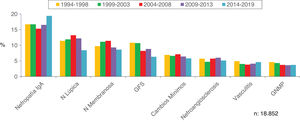
| 15−65 | 4107 (71.8) | 3355 (70.5) | 4236 (73.0) | 3683 (70.6) | 3471 (67.1) | 18,852 (70.7) |
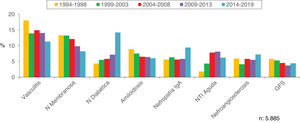
| 65−80 | 1058 (18.5) | 1012 (21.3) | 1252 (21.6) | 1232 (23.6) | 1331 (25.7) | 5885 (22.1) |
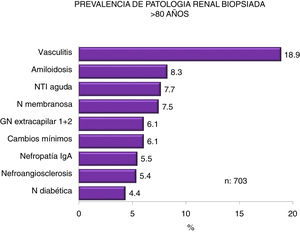
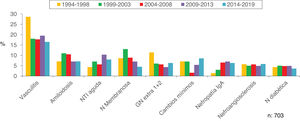
| > 80 | 70 (1.2) | 100 (2.1) | 124 (2.1) | 185 (3.5) | 224 (4.3) | 703 (2.6) |
| Total | 5722 | 4759 | 5800 | 5216 | 5174 | 26,671 ** |
*Percentage with respect to total in each period of time.
**Total number of cases in groups of age documented in the registry.
The median ages and creatinine at the time of the renal biopsy of the entire population are 50 years and 1.6 mg / dl, respectively (neither one have a Gaussian distribution). Males predominate over females, with a ratio of 1.5. The prevalence of hypertension is 55%. In Table 3 these values are given for each age group.
Age, gender, hypertension and renal function according to age groups.
| Age group (years) | Age (years) (median) * | Quotient Male/ Female | Hypertension (%) | Serum creatinine (median, mg / dl) * |
|---|---|---|---|---|
| < 15 (n = 1.231) | 10 | 1.3 | 19 | 0.6 |
| 15−65 (n = 18,852) | 44 | 1.5 | 52 | 1.4 |
| 65−80 n = 5.885) | 72 | 1.6 | 69 | 2.5 |
| > 80 (n = 703) | 83 | 1.3 | 69 | 3.2 |
| Total (n ° = 26.671) | 50 | 1.5 | 55 | 1.6 |
* Non-Gaussian distribution.
Regarding the study method, 74.7% of the biopsies had a simultaneous study with an optical microscope and immunofluorescence, 17.8% also had electron microscopy, while 7% only had a study with optical microscopy and 0.5% only immunofluorescence.
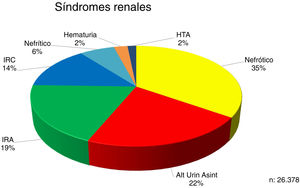
The proportions of the renal syndromes at the time of the renal biopsy are indicated in Fig. 1.
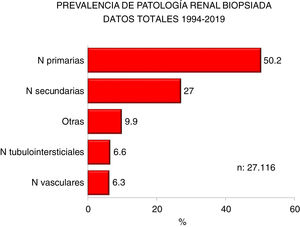
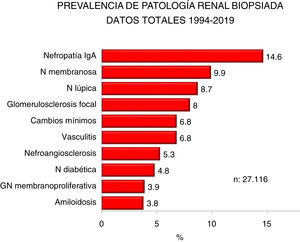
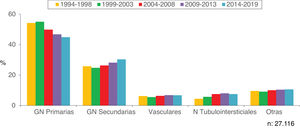
Figs. 2 and 3 show the overall prevalence of all grouped kidney biopsies and for specific diagnoses, respectively.
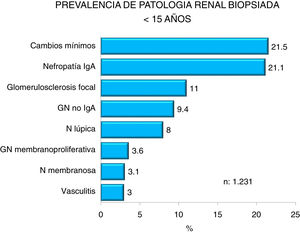
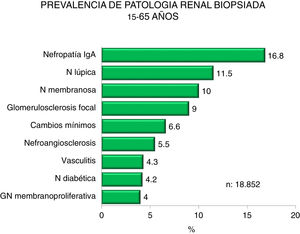
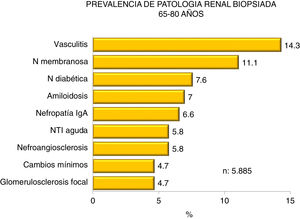
Figs. 4–7 summarize the prevalence in the different age ranges.
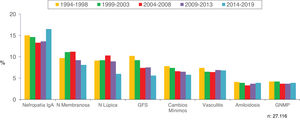
Figs. 8 and 9 show the trend of the most frequent kidney pathologies, grouped and by histological diagnoses, respectively.
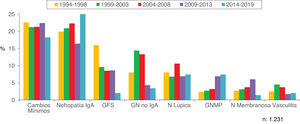
Figs. 10–13 show the trends of the biopsied pathologies in each age interval, <15 years, 15-65 years, 65-80 years and > 80 years, respectively.
Tables 4 and 5 summarize the conclusions of all these results.
Summary of data from the Glomerulonephritis Registry (1994-2019). General data.
| Prevalence of males in all age groups |
| The median age is 50 years old |
| High presence of hypertension and kidney failure in adults and the elderly |
| Nephrotic syndrome is the most frequent indication for kidney biopsy, followed by asymptomatic urinary abnormalities. |
| Half of all diagnoses are primary kidney diseases |
| IgA, membranous and lupus nephropathy are the most frequent diagnosis (1/3 of the total) |
| There are changes in the temporal trends of diagnoses in general: |
| -Increase of secondary GN and tubulointerstitial nephropathies and decrease of primary GN |
| -Increased prevalence of IgA nephropathy and decreased of glomerulosclerosis, minimal changes, membranous and lupus nephropathy. Steadiness of vasculitis, amyloidosis and membranoproliferative GN |
Summary of data from the Glomerulonephritis Registry (1994-2019). Data and trends according to age groups.
| <15 years: |
| Predominance of minimal change nephropathy, IgA nephropathy and focal glomerulosclerosis. |
| Increased in IgA nephropathy, membranoproliferative GN and decreased prevalence of minimal changes and glomerulosclerosis. |
| 15−65 years : |
| Predominance of IgA, lupus and membranous nephropathy. |
| Increasing IgA nephropathy and decreasing glomerulosclerosis and lupus. |
| 65−80 years : |
| Predominance of vasculitis, diabetic and membranous nephropathy. |
| Increasing IgA nephropathy, diabetic nephropathy, and acute interstitial nephritis, with a decrease in vasculitis, membranouse GN, amyloidosis, and glomerulosclerosis; nephroangiosclerosis unchanged. |
| > 80 years : |
| Predominance of vasculitis, amyloidosis, and acute tubulointerstitial nephritis. |
| Increase in acute interstitial nephritis, minimal changes and IgA nephropathy, with a decrease in vasculitis and membranous GN and without changes in nephroangiosclerosis and diabetic nephropathy. |
Nephrology has undergone numerous changes in recent years and kidney diseases that require biopsy have not been unaffected by these modifications. The emergence of new entities (C3 GN, among others) stands out, as well as a new approach for the classification and reporting GN.147,148 Therefore, the registries must adapt to these changes in order to collect the data in a uniform and modern manner. Without a doubt, the REGN needs some changes such as:
- a)
Modification of the data completion sheet, with an extension of the clinical syndromes that indicate the reason to perform the renal biopsy (eg: nephrotic syndrome + acute renal failure; urinary alterations + hypertension).
- b)
Possibility of selecting various pathologies detected in the renal biopsy (eg: IgA N + nephroangiosclerosis; diabetic nephropathy + extracapillary GN).
- c)
Improve data collection to find out the incidence of the different pathologies “p.m.p”. according to the reference population of the center performing the kidney biopsies.
- d)
Adapt the classification of kidney disease according to the new system proposed at the Mayo Clinic.147
- e)
Increase participation, especially in Child Nephrology Centers.
- f)
Promote multicenter studies and perform studies or clinical trials based on the data obtained.
- g)
Maintain the analysis of the data and its subsequent publication, to determine changes in prevalence and incidence, as well as to evaluate correlations between clinical syndromes and the histological substrate.
- h)
Incorporate information on associated morbidity, in order to establish the diagnosis of secondary forms (eg, viral or other infections, tumors, among others), given the availability of new biomarkers.78
Finally, the REGN must remain active to maintain the achievements, accumulated experience and number of biopsies. Since it is one of the largest and most reliable registries in the world, it must continue to provide data for the improvement of clinical practice and for the development of clinical research. Knowing the quality and quantity of participating centers - despite the absence of some of them - it is predictable that the activity will not decline in the coming years.
Participating Hospitals and the City (in alphabetical order).
| Ciudad Sanitaria Virgen de la Nieves (Granada) |
| Clínica Quirón (Madrid) |
| Clínica Ruber (Madrid) |
| Clínica Universidad de Navarra (Navarra) |
| Clínica Vistahermosa (Alicante) |
| Complejo Asistencial de Palencia (Palencia) |
| Complejo Asistencial Universitario de Burgos (Burgos) |
| Complejo Asistencial Universitario de León (León) |
| Complejo Asistencial Universitario de Salamanca (Salamanca) |
| Complejo Hospitalario Arquitecto Marcide Novoa Santos Ferrol (A Coruña) |
| Complejo Hospitalario de Navarra (Pamplona) |
| Complejo Hospitalario Nuestra Sra. de la Candelaria (Tenerife) |
| Complejo Hospitalario Princesa de España (Jaén) |
| Complejo Hospitalario San Millán y San Pedro (La Rioja) |
| Complejo Hospitalario San Pedro de Alcántara (Cáceres) |
| Complejo Hospitalario Universitario A Coruña (A Coruña) |
| Complejo Hospitalario Universitario de Santiago (A Coruña) |
| Complejo Hospitalario Universitario Reina Sofía (Córdoba) |
| Complejo Universitario San Carlos (Madrid) |
| Consorci Corporació Sanitaria Parc Taulí de Sabadell (Barcelona) |
| Consorci Hospitalari de Terrassa (Barcelona) |
| Consorcio Hospital General Universitario de Valencia (Valencia) |
| Fundació Althaia Manresa (Barcelona) |
| Fundación Hospital Alcorcón (Madrid) |
| Fundación Hospital Manacor (Mallorca) |
| Fundación Puigvert (Barcelona) |
| Hospital 12 de Octubre Infantil (Madrid) |
| Hospital Arnau de Vilanova (Valencia) |
| Hospital Central de Asturias Infantil (Asturias) |
| Hospital Clinic i Provincial (Barcelona) |
| Hospital Clínico Universitario de Valencia (Valencia) |
| Hospital Clínico Universitario de Valladolid (Valladolid) |
| Hospital Clínico Universitario de Zaragoza (Zaragoza) |
| Hospital Clínico Universitario Virgen de la Victoria (Málaga) |
| Hospital Comarcal de Melilla (Melilla) |
| Hospital Comarcal Francesc De Borja (Valencia) |
| Hospital Costa del Sol Marbella (Málaga) |
| Hospital Cristal Piñor (Orense) |
| Hospital de Basurto (Bilbao) |
| Hospital de Bellvitge (Barcelona) |
| Hospital de Cruces (Bilbao) |
| Hospital de Cruces Infantil (Bilbao) |
| Hospital de Jerez (Cádiz) |
| Hospital de la Cruz Roja (Madrid) |
| Hospital de la Santa Creu i Sant Pau (Barcelona) |
| Hospital de León (León) |
| Hospital de Manises (Valencia) |
| Hospital de Navarra (Pamplona) |
| Hospital de Palamós (Gerona) |
| Hospital de Poniente El Ejido (Almería) |
| Hospital de Sagunto (Valencia) |
| Hospital de San Agustín Avilés (Asturias) |
| Hospital de Torrevieja Alicante (Alicante) |
| Hospital de Villajoyosa-Benidorm (Alicante) |
| Hospital de Zumárraga (Guipúzcoa) |
| Hospital del Aire (Madrid) |
| Hospital del Henares (Madrid) |
| Hospital del Mar (Barcelona) |
| Hospital del Mollet (Barcelona) |
| Hospital del Sureste (Madrid) |
| Hospital del Tajo (Madrid) |
| Hospital General de Elda (Alicante) |
| Hospital General de Especialidades Ciudad de Jaén (Jaén) |
| Hospital General de Granollers (Barcelona) |
| Hospital General de la Marina Alta de Denia (Alicante) |
| Hospital General de Segovia (Segovia) |
| Hospital General de Soria (Soria) |
| Hospital General Universitario de Albacete (Albacete) |
| Hospital General Universitario de Alicante (Alicante) |
| Hospital General Universitario de Castellón (Castellón) |
| Hospital General Universitario de Ciudad Real (Ciudad Real) |
| Hospital General Universitario de Elche (Alicante) |
| Hospital General Universitario de Guadalajara (Guadalajara) |
| Hospital General Universitario de Málaga Adultos (Málaga) |
| Hospital General Universitario de Málaga Infantil (Málaga) |
| Hospital General Universitario Gregorio Marañón Adultos (Madrid) |
| Hospital General Universitario Gregorio Marañón Infantil (Madrid) |
| Hospital Gral. Univ. Reina Sofía (Murcia) |
| Hospital GU Santa Lucía de Cartagena Infantil (Murcia) |
| Hospital Infanta Cristina (Madrid) |
| Hospital Infanta Elena (Madrid) |
| Hospital Infanta Leonor (Madrid) |
| Hospital Infanta Sofía (Madrid) |
| Hospital Militar de Zaragoza (Zaragoza) |
| Hospital Militar Gómez Ulla (Madrid) |
| Hospital Montecelo (Pontevedra) |
| Hospital Nuestra Señora de Sonsoles (Ávila) |
| Hospital Nuestra Señora del Prado de Talavera (Toledo) |
| Hospital Obispo Polanco (Teruel) |
| Hospital Provincial de Pontevedra (Pontevedra) |
| Hospital Público Lluis Alcanys de Xátiva (Valencia) |
| Hospital Público Virgen de los Lirios (Alicante) |
| Hospital Rafael Méndez (Murcia) |
| Hospital Regional de Málaga Infantil (Málaga) |
| Hospital Rey Juan Carlos, Móstoles (Madrid) |
| Hospital San Joan de Deu (Barcelona) |
| Hospital San Jorge (Huesca) |
| Hospital Santa María del Rosell (Murcia) |
| Hospital Santiago Apóstol (Vitoria) |
| Hospital Son Llàtzer (Mallorca) |
| Hospital Universitari de Girona Doctor Josep Trueta (Gerona) |
| Hospital Universitari Germans Trias i Pujol (Barcelona) |
| Hospital Universitario 12 de Octubre Adultos (Madrid) |
| Hospital Universitario Araba (Álava) |
| Hospital Universitario Central de Asturias (Oviedo) |
| Hospital Universitario de Badajoz (Badajoz) |
| Hospital Universitario de Cabueñes Gijón (Asturias) |
| Hospital Universitario de Canarias (Tenerife) |
| Hospital Universitario de Fuenlabrada (Madrid) |
| Hospital Universitario de Galdakao (Vizcaya) |
| Hospital Universitario de Getafe (Madrid) |
| Hospital Universitario de Gran Canaria Dr. Negrín (Gran Canaria) |
| Hospital Universitario de Granada (Granada) |
| Hospital Universitario de la Princesa (Madrid) |
| Hospital Universitario de Puerto Real (Cádiz) |
| Hospital Universitario Doctor Peset (Valencia) |
| Hospital Universitario Donostia (Guipúzcoa) |
| Hospital Universitario Fundación Jiménez Díaz (Madrid) |
| Hospital Universitario Infantil Niño Jesús (Madrid) |
| Hospital Universitario Insular de Las Palmas (Gran Canaria) |
| Hospital Universitario Joan XXIII (Tarragona) |
| Hospital Universitario Juan Ramón Jiménez (Huelva) |
| Hospital Universitario La Fe Adultos (Valencia) |
| Hospital Universitario La Fe Infantil (Valencia) |
| Hospital Universitario La Paz (Madrid) |
| Hospital Universitario La Paz Infantil (Madrid) |
| Hospital Universitario Lucus Augusti (Lugo) |
| Hospital Universitario Marqués de Valdecilla (Santander) |
| Hospital Universitario Miguel Servet (Zaragoza) |
| Hospital Universitario Miguel Servet Infantil (Zaragoza) |
| Hospital Universitario Nuestra Señora de Candelaria (Tenerife) |
| Hospital Universitario Nuestra Señora de Valme (Sevilla) |
| Hospital Universitario Príncipe de Asturias (Madrid) |
| Hospital Universitario Puerta de Hierro (Madrid) |
| Hospital Universitario Puerta del Mar (Cádiz) |
| Hospital Universitario Ramón y Cajal (Madrid) |
| Hospital Universitario Ramón y Cajal Infantil (Madrid) |
| Hospital Universitario Río Hortega (Valladolid |
| Hospital Universitario San Agustín (Oviedo) |
| Hospital Universitario Severo Ochoa (Madrid) |
| Hospital Universitario Son Espases (Mallorca) |
| Hospital Universitario Torrecárdenas (Almería) |
| Hospital Universitario Vall d'Hebrón (Barcelona) |
| Hospital Universitario Vall Hebrón Infantil (Barcelona) |
| Hospital Universitario Virgen de la Arrixaca (Murcia) |
| Hospital Universitario Virgen del Rocío (Sevilla) |
| Hospital Universitario Virgen Macarena (Sevilla) |
| Hospital Valle Del Nalón (Asturias) |
| Hospital Virgen de la Concha (Zamora) |
| Hospital Virgen de la Luz (Cuenca) |
| Hospital Virgen de la Salud (Toledo) |
| Hospital Virgen del Rocío Infantil (Sevilla) |
| Hospital Vithas Xanit Internacional Benalmádena (Málaga) |
| Hospital Xeral Cíes (Pontevedra) |
| Policlínica Miramar (Mallorca) |
| Policlínico de Vigo, SA (Pontevedra) |
| Sanatorio Perpetuo Socorro (Alicante) |
Please cite this article as: López-Gómez JM, Rivera F. Registro de glomerulonefritis de la Sociedad Española de Nefrología en 2019: pasado, presente y nuevos retos. Nefrologia. 2020;40:371–383.