The PIBHE study, promoted by the Spanish Liver and Kidney Association and the Dialysis Virus Group of the Spanish Society of Nephrology, is the first study to determine the status of haemodialysis patients with chronic HBV infection and the immunisation against the vaccine.
MethodThe study has a national multicentre, observational, cross-sectional design and was carried out between January 2013 and 2014. A data collection folder was sent to all the nephrology departments and outpatient haemodialysis units in Spain, to be completed based on patient medical files after informed consent. The data were recorded in a central database.
ResultsA total of 215 centres participated (15,645 patients), with an HBV prevalence of 1.03%. HCV or HIV was present in 7.2% of the HBV(+) patients. Viral load was below 2000IU/ml in 80%. GOT and GPT levels were 19.1±10.1 and 15.9±9.6IU/ml, respectively. Liver biopsy was performed in 7.1%. Antiviral treatment was prescribed in 30% and suspended in 12.5%: entecavir (13.3%), lamivudine (10%), adefovir and tenofovir (6.7%), and interferon (3.3%). A total of 34.5% were candidates for renal transplantation and 6.9% had not been evaluated; 64.3% were followed up by a gastroenterologist; 27.2% of HBV(−) patients without immunisation had not been vaccinated. Fourteen different immunisation schedules had been used, with an immunisation rate of 58.8%. Mean anti-HBs stood at 165.7±297.8mIU/ml. A total of 72.7% of patients had received a vaccination course; 26.4%, 2 cycles; 1.0%, 3 cycles; and 11.6%, a booster dose. A total of 28.3% had a poor response (anti-HBs 10–99mIU/ml); 22.4%, an optimal response (anti-HBs 100–999mIU/ml); and 7.9%, an excellent response (anti-HBs ≥1000mIU/ml). Age was significantly associated with response to vaccination; the mean age of nonresponders was significantly higher than patients who had a response of any kind (p<0.05). The highest probability of an immune response was achieved with 4 doses of 40mcg of adjuvanted vaccine (OR: 7.3; 95%CI 3.4–15.7), for the same age and number of cycles and boosters. Age, adjuvanted vaccine, dose and vaccination schedule influenced the immune response and the anti-HBs titres reached (p<0.05).
ConclusionThe prevalence of chronic HBV infection in haemodialysis in Spain is low and so are the rates of immunisation against the virus. The vaccination schedules used are very diverse and have been observed to correlate with the immune response. It would therefore be necessary to establish a protocol for the most effective vaccination schedule to increase immunisation in these patients.
El estudio PIBHE, promovido por la Asociación Española de Hígado y Riñón y el Grupo de Virus en Diálisis de la Sociedad Española de Nefrología, es el primer estudio que determina la situación de los pacientes en hemodiálisis con infección crónica por el VHB y la inmunización frente a la vacuna.
MétodoEstudio nacional multicéntrico, observacional, de corte transversal, entre enero de 2013 y de 2014. Se envió un cuaderno de recogida de datos a todos los servicios de nefrología y unidades extrahospitalarias de hemodiálisis de España, para que lo cumplimentaran a partir de la historia clínica del paciente, tras consentimiento informado. Los datos se incluyeron en una base central.
ResultadosParticiparon 215 centros (15.645 pacientes), con una prevalencia del VHB del 1,03%. El 7,2% de los pacientes VHB(+) estaba coinfectado por el VHC o VIH. La carga viral era inferior a 2.000 UI/ml en el 80%. Los niveles de GOT y GPT fueron de 19,1±10,1 y 15,9±9,6 UI/ml, respectivamente. La biopsia hepática se había realizado en el 7,1%. El 30% había recibido tratamiento antiviral, que se había suspendido en el 12,5%. El más empleado había sido entecavir (13,3%), seguido de lamivudina (10%), adefovir y tenofovir (6,7%) e interferón (3,3%). El 34,5% era candidato a trasplante renal y el 6,9% no había sido evaluado. Se encontraban en seguimiento por un digestólogo el 64,3%. No había sido vacunado el 27,2% de los pacientes VHB(−) sin inmunización. Se emplearon 14 pautas distintas de vacunación, con un 58,8% de inmunización. La media de anti-HBs se situaba en 165,7±297,8mUI/ml. El 72,7% de los pacientes había recibido un ciclo de vacunación; el 26,4%, 2 ciclos; el 1,0%, 3 ciclos y el 11,6%, una dosis de recuerdo. El 28,3% tuvo una respuesta pobre (anti-HBs 10-99mUI/ml); el 22,4%, una respuesta óptima (anti-HBs 100-999mUI/ml); y el 7,9%, una respuesta excelente (anti-HBs≥1.000mUI/ml). La edad se asoció significativamente con la respuesta a la vacunación, de manera que los pacientes que no respondieron tenían una edad media significativamente mayor que los pacientes que obtuvieron cualquier tipo de respuesta (p<0,05). La mayor probabilidad de conseguir una respuesta inmunitaria se alcanzaba con 4 dosis de 40mcg de vacuna adyuvada (OR: 7,3; IC 95%: 3,4-15,7), a igualdad de edad y número de revacunaciones y recuerdos. La edad, la vacuna adyuvada, la dosis y el esquema de vacunación influían en la respuesta inmunitaria y en el título de anti-HBs alcanzado (p<0,05).
ConclusiónLa prevalencia de la infección crónica por el VHB en hemodiálisis en España es baja, así como las tasas de inmunización frente a este virus. Los esquemas de vacunación empleados son muy diversos y se han correlacionado con la respuesta inmunitaria, por lo que sería necesario protocolizar la pauta más eficaz para aumentar la inmunización en estos pacientes.
Hepatitis B virus (HBV) infection is more frequent in patients with chronic renal disease (CRD) than in the general population. In haemodialysis patients, the prevalence ranges from 0% to 10%,1 with a wide variance between the country's haemodialysis units. In Spain, in 2003 the prevalence of HBV infection in haemodialysis patients was estimated to be 3.1%.1 In kidney transplant patients, the prevalence of HBV infection was around 2%,2 also with a wide geographic and demographic variability.
The infection by HBV in this population has decreased since the application of prevention measures in the 1970s, and the vaccination in 1986.
The natural history of hepatitis B in the dialysis population is not well understood due to its slow progression, which may be caused by numerous factors, including coinfection with hepatitis C virus, alcohol consumption and immunosuppression. In kidney transplant patients, chronic hepatitis B progresses more aggressively than in dialysis patients; this is due to the immunosuppression and the reduced activity of cytotoxic T lymphocyte, which increase viral replication.3 Dialysis patients rarely present symptoms of acute hepatitis B and their transaminase levels are usually normal or mildly elevated during chronic infection.4,5 These differences may be explained by uraemia, vitamin B deficiency and the patient's nutritional status. The amount of HBV(+) in dialysis patients is usually low and stable over time.6
The main objective in the treatment chronic HBV infection is the clearance of HBsAg and the seroconversion to anti-HBs. However, this is only achieved in a minority of immunocompetent patients and is rarely seen in immunocompromised patients. A more realistic objective is to suppress HBV replication efficiently and persistently to reduce hepatic necroinflammatory activity and halt or delay fibrosis progression to prevent the development of complications such as cirrhosis, decompensation of liver function and hepatocellular carcinoma.
HBV infection treatment indications are based on viral load, transaminase levels and severity of liver failure. The dosage of all drugs given to patients with chronic kidney disease (CKD) must be adjusted and has to be administered with caution, serum creatinine and, in some cases, serum phosphorus levels should be monitorized. The use of interferon is contraindicated in kidney transplant patients due to risk of rejection.
Universal vaccination of CKD patients, patients on dialysis and healthcare professionals are recommended. The most recommended regimen is the double dose (40mcg) of the conventional vaccine (Engerix®, HBvaxPRO®) at 0, 1, 2 and 6 months.7 The immunity rates of haemodialysis patients vaccinated against HBV range from 40% to 70%, compared to 97% for the general population,8 with a subsequent progressive loss of immunisation that increases with time. Various adjuvants were studied to improve the immunisation in specific populations (granulocyte and macrophage colony-stimulating factor, interleukin-2, interferon, thymopentin),9,10 until a vaccine containing 3-O-desacyl-40-monophosphoryl lipid A was developed. A clinical trial by Tong et al.11 compared immunisation with the conventional double-dose vaccine against the single-dose adjuvanted vaccine (Fendrix®) at 0, 1, 2 and 6 months, finding immunity rates of 84% versus 91%. A number of factors affect the response to HBV vaccination: anaemia, malnutrition,12–14 weight, male gender,15,16 renal function, secondary hyperparathyroidism, reduced immunoglobulin production, loss of interleukin-2 by T lymphocytes, macrophage dysfunction and high levels of indoleamine 2,3-dioxygenase.17
It is important to develop strategy for HBV infection prevention and management in the CRD population because of their high exposure to blood or blood-derived products, frequent venipuncture, invasive procedures, medical equipment and healthcare personnel.
There is lack of data in the medical literature concerning evaluation and monitoring of chronic hepatitis B in haemodialysis patients and the level of immunisation and the vaccination in patients without hepatitis B. Thus we believe it was necessary to conduct a cross-sectional study to encompass all associated aspects and to detect improvements in disease management, as well as multidisciplinary intervention with nephrologists and hepatologists. This study, sponsored by the Spanish Liver and Kidney Association (AEHR) and the Dialysis Virus Group of the Spanish Society of Nephrology (SEN), is the first of its kind to incorporate the following objectives: (1) to determine the prevalence of chronic HBV infection in CRD patients undergoing renal replacement therapy with haemodialysis in Spain, and to evaluate liver disease-related aspects and their status in regard with kidney transplant; (2) to gain information about the immunisation against HBV in this population and the associated response factors.
MethodA formulary comprising the study variables to be completed for every haemodialysis patient using their medical records. This was sent together with a cover letter via regular mail and also by email to every nephrology department and every outpatient haemodialysis unit in Spain. This was, followed by a telephone call to confirm the reception of the documentation. This procedure was repeated 6 months later in those sites that did not respond. The case report form had to be completed by a local investigator from each site and be returned by postmail to the coordinating site, or by email to the study coordinator group. Then, the data was entered into a central database.
Patients who had not previously granted their authorisation for the use of their data in scientific research, stored by the healthcare site in question, had to sign an informed consent to authorise the use of their data.
The study was approved by the Independent Ethics Committee of the coordinating site, Hospital La Mancha-Centro in Alcázar de San Juan (Ciudad Real), Spain.
Data collection was conducted from January 2013 to January 2014, followed by centralisation and analysis.
Variables- •
Site
- •
Number of patients at the site, number of HBV(+) patients
- •
Investigator
- •
Date
- •
HBV(+) patients
- ∘
Patient's initials
- ∘
Age
- ∘
HCV/HIV coinfection (yes/no)
- ∘
Viral load
- ∘
GOT/GPT
- ∘
GGT
- ∘
- •
Liver biopsy (yes/no, description)
- ∘
Previous HBV treatment (interferon, lamivudine, entecavir, tenofovir, adefovir, others)
- ∘
Treatment duration
- ∘
Dose
- ∘
Discontinuation (yes/no, cause)
- ∘
Candidate for kidney transplant (yes/no/not evaluated, refused by the patient)
- ∘
Follow-up by the Gastrontestinal (GI) service (yes/no)
- ∘
- •
HBV(−) patients
- ∘
Serology: anti-HBs antibodies, anti-HBc antibodies
- ∘
HBV vaccination (yes/no)
Type of vaccine (Engerix®, Fendrix®)
Regimen (0-1-6, 0-1-2-6, other)
Dose
Booster (yes/no), type of vaccine, regimen, dose
- ∘
- •
Did this questionnaire help you to assess HBV infection management in your unit? (yes/no)
Measurement of central tendency (mean and median) was used to describe the quantitative variables, together with measures of dispersion (standard deviation or interquartile range), depending on normal distribution. The qualitative variables were described by their absolute and relative frequencies.
The chi-square test and Student's t-test were used to compare qualitative variables, and the Mann–Whitney U test was used to compare the means of quantitative variables. A multivariate logistic regression model was used to assess the association between immunisation and the different therapeutic regimens. p<0.05 was considered to be statistically significant.
The data were analysed using version 18.0 of the SPSS program.
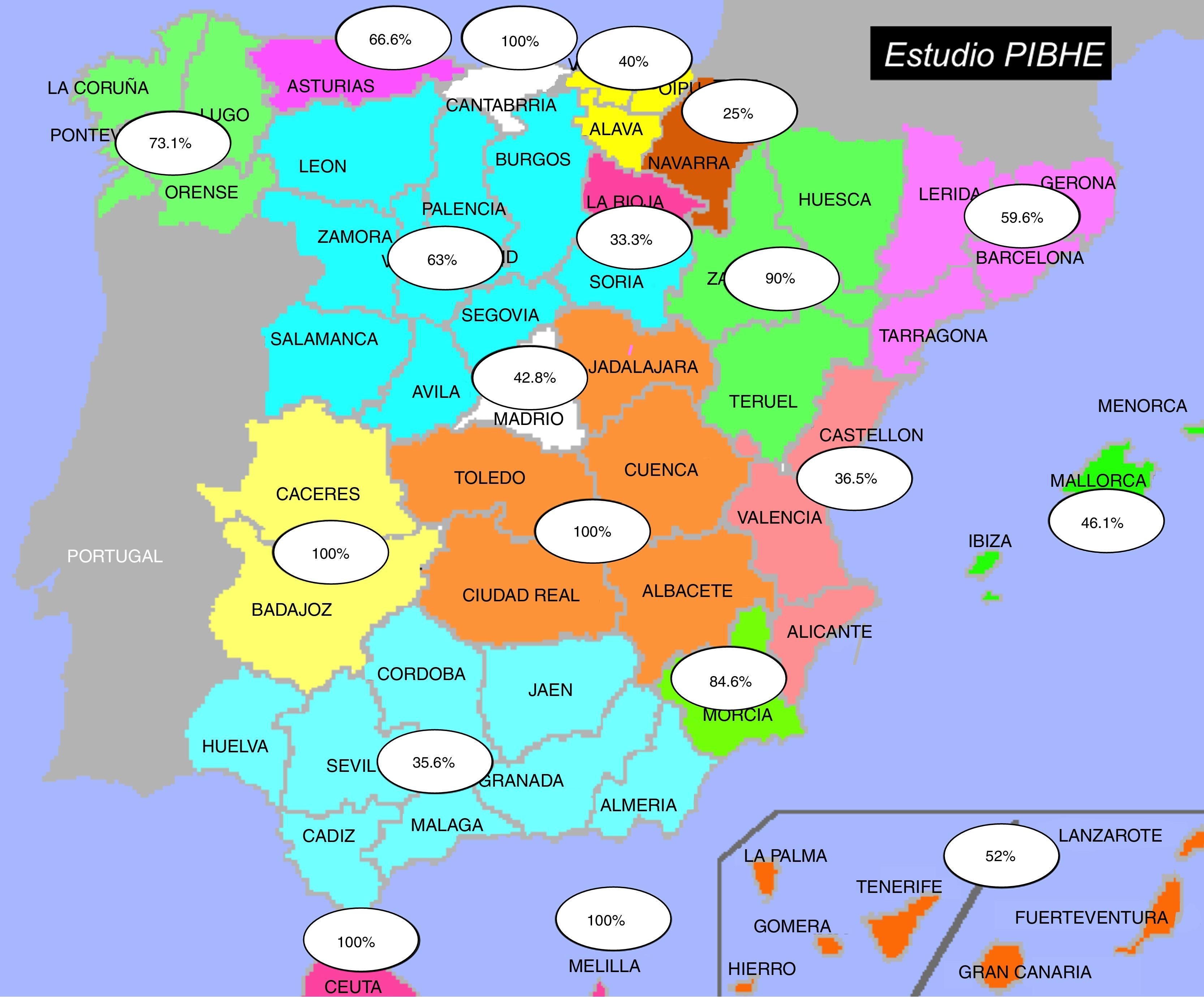
ResultsData of the participating sitesOut of the 366 haemodialysis units in Spain, 215 (59%) participated in the study. 53.7% of the participating units were at hospitals. All units sent their prevalence data and 42 sent the completed case report form. Fig. 1 shows the participation distributed by autonomous region.
One hundred and sixty-two out of the 15,645 registered patients were HBV(+), so the prevalence of hepatitis B is 1.03%. Based on the sample surveyed, 64.2% of sites had at least one HBV(+) patient.
Case report forms for 2187 patients were received.
Information about HBV(+) patientsHBV(+) patients had a mean age of 50±14.6 years, ranging from 26 to 66 years.
7.2% were coinfected with hepatitis C virus or human immunodeficiency virus (HIV). 19.2% had anti-HBe antibodies and this had not been evaluated in 3.8% of cases. Viral quantification was below 2000IU/ml in 80% of patients. GOT and GPT levels were 19.1±10.1 and 15.9±9.6IU/ml, respectively. 7.1% of patients had undergone liver biopsy.
30% of patients had received antiviral treatment, which was suspended in 12.5% of cases. The most-prescribed antiviral was entecavir (13.3%), followed by lamivudine (10%), adefovir and tenofovir (both 6.7%), interferon (3.3%) and others (3%).
34.5% of patients were candidates for kidney transplant and 6.9% had not been evaluated. 64.3% were being followed up by a gastroenterologist.
Information on HBV(−) patientsHBV(−) patients had a mean age of 66.8±14.9 years, ranging from of 19 to 100 years.
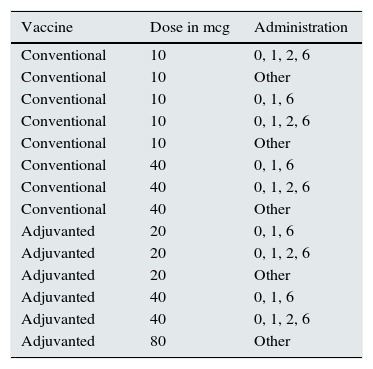
A 27.2% of patients without immunisation had not been vaccinated; 17.9% of patients had anti-HBc antibodies, and 66.1% of these had not been vaccinated. Fourteen different vaccination regimens were used; these are shown in Table 1. A 58.8% of vaccinated patients were successfully immunised. Mean of anti-HBs was 165.7±297.8mIU/ml (0–1000).
Vaccination regimens.
| Vaccine | Dose in mcg | Administration |
|---|---|---|
| Conventional | 10 | 0, 1, 2, 6 |
| Conventional | 10 | Other |
| Conventional | 10 | 0, 1, 6 |
| Conventional | 10 | 0, 1, 2, 6 |
| Conventional | 10 | Other |
| Conventional | 40 | 0, 1, 6 |
| Conventional | 40 | 0, 1, 2, 6 |
| Conventional | 40 | Other |
| Adjuvanted | 20 | 0, 1, 6 |
| Adjuvanted | 20 | 0, 1, 2, 6 |
| Adjuvanted | 20 | Other |
| Adjuvanted | 40 | 0, 1, 6 |
| Adjuvanted | 40 | 0, 1, 2, 6 |
| Adjuvanted | 80 | Other |
In 23% of patients the type vaccine administered was not documented. Engerix® was administered to 52.3% of patients, followed by Fendrix® in 26.9% and HBvaxPRO® in 20.9%.
72.7% of patients received one vaccination cycle; 26.4% received 2 cycles; 1.0%, 3 cycles; and 11.6% of patients were given a booster. The immunisation relative to the number of cycles was 80% with one cycle, 19.5% with 2 cycles and 0.5% with 3 cycles.
The most used booster vaccination was Engerix® (39.3%) followed by Fendrix® (32.5%) and HBvaxPRO® (24.0%). The type of booster vaccination administered was unknown in 3.3% of patients.
Vaccination responseA 41.4% of patients did not respond to the vaccination. A 28.3% had a poor response (anti-HBs 10–99mIU/ml); A 22.4% had an optimal response (anti-HBs 100–999mIU/ml); and 7.9% had an excellent response (anti-HBs ≥1000mIU/ml).
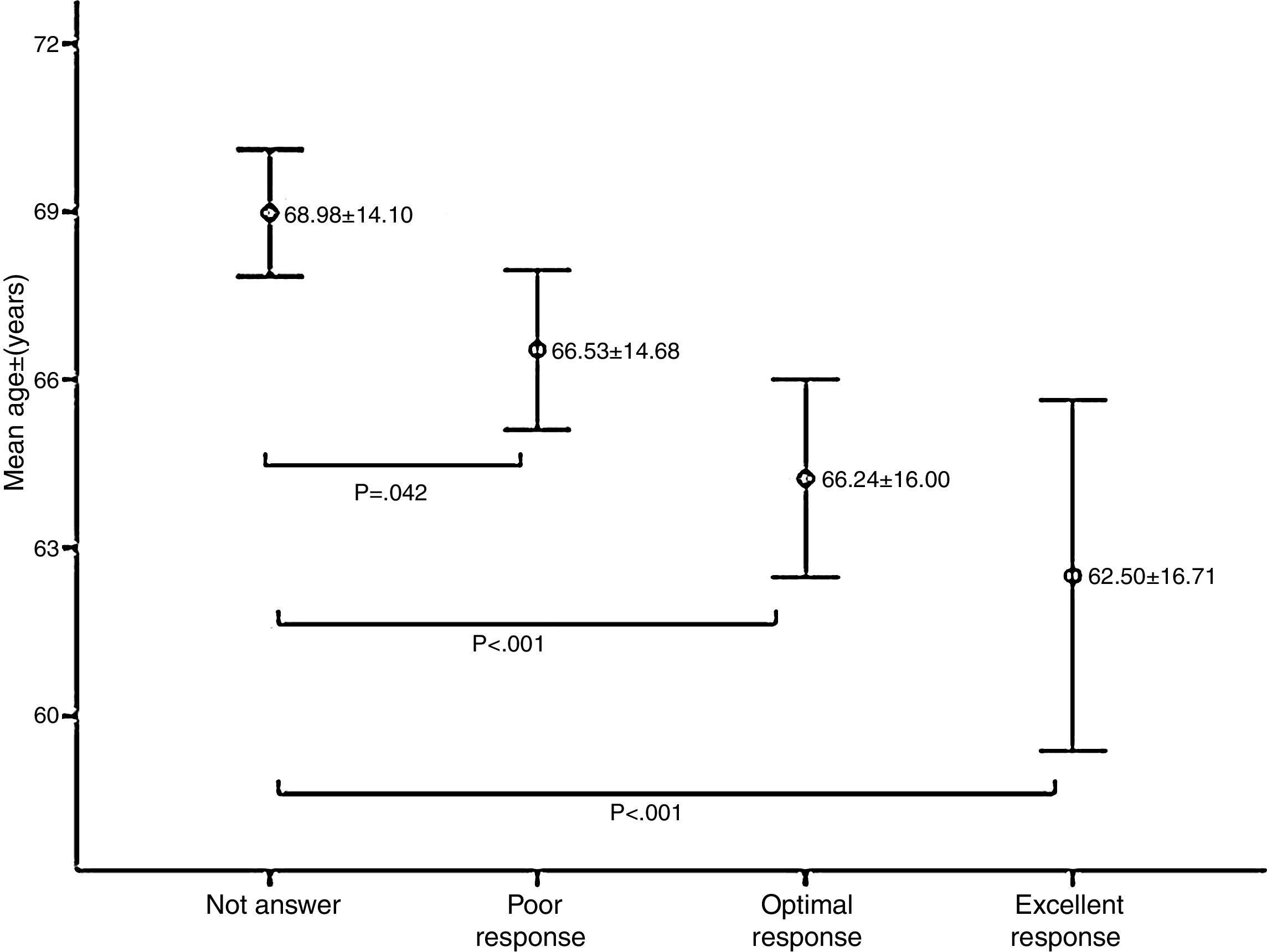
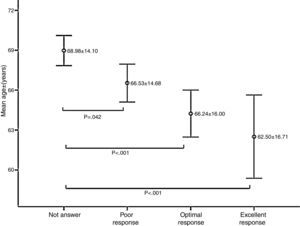
Age was significantly associated with response to vaccination; the mean age of non-responders was significantly higher than patients who had a response of any kind (p<0.05) (Fig. 2).
The highest likelihood of achieving an immune response was obtained with 4 doses of 40mcg of adjuvanted vaccine (OR: 7.3; 95%CI: 3.4–15.7), for the same age and number of cycles and boosters. Age, adjuvanted vaccine, dose and vaccination regimen influenced the immune response and the anti-HBs titres achieved (p<0.05).
Questionnaire self-assessmentA 78% of the representative investigators from each site deemed the questionnaire to have been helpful in assessing patient management at their unit. Regarding to HBV infection and immunisation, a 17.1% did not find the questionnaire useful and 4.9% did not answer.
DiscussionData from the present study reveals that the prevalence of chronic hepatitis B in haemodialysis patients in Spain to be 1.03%, a value lower than the 3.1% estimated by Burdick et al. in the 2003 DOPPS study.1 This reduction in the prevalence of HBV in haemodialysis patients in Spain could be due to numerous reasons, including the death of patients infected by contaminated transfusions prior to the 1990s and the preventive measures implemented by the haemodialysis units including isolation and vaccination. The DOPPS study included 308 sites but only 20 were from Spain; this is in contrast to the 215 sites recruited to the PIBHE study, which was not free of sampling bias given that HBV(+) patients are more centralised than HCV(+). This is evidenced by the fact that 64.5% of sites had at least one HBV(+) patient, versus 75.9% of sites with HCV(+), as shown reflected in the SHECTS study.18
Patients with HBV(+) were younger than HBV(−) patients, with a mean age of 50±14.6 versus 66.8±14.9 years, respectively, which may be related to shorter life expectancy due to the infection. Although no HBV morbidity/mortality studies in CKD or dialysis patients have been conducted to date. In HBV+kidney transplant patients, mortality at 10 and 20 years is 85% and 71%, respectively versus 98% and 95% in HBV(−) transplant patients, despite antiviral treatment.19 A meta-analysis by Fabrizi et al.20 found that the post-transplant mortality risk of kidney transplant patients with HBV infection is 2.49 times greater than for non infected patients.
Liver biopsy is not widely used (7.1%). This may be due to the increased risk of bleeding in kidney patients and also the development of the elastography, although this technique has yet to be validated in this population.
The nephrotoxicity associated with tenofovir and adefovir may explain the greater use of entecavir and lamivudine, as reflected in the antiviral prescription data. One of the reasons why antivirals were prescribed in only 30% of patients may be because of to the situation of inactive carriers of HBV, with low viral replication and normal transaminase levels.
Although this study shows that most HBV(+) patients are assessed for kidney transplant, it is not known why the proportion of candidates is low (34.5%).
Follow-up of all patients by a gastroenterologist is advisable given the risk of HBV reactivation and hepatocellular carcinoma. However, this study suggests that such follow-up only occurs in 64.3% of cases.
With respect to the HBV(−) patients, it is of note that 23% had not been vaccinated, despite the fact that the clinical guidelines recommend vaccination for all haemodialysis patients.
A 17.3% of patients have anti-HBc antibodies, but a considerable proportion lack immunisation and have not been vaccinated (33.8%); these patients are also more likely to achieve immunity by immunological memory, and thus are at greater risk of reactivation in the event of immunosuppression, making vaccination even more necessary.
The overall number of immunised haemodialysis patients is 61.9%, similar to previous studies, 68.7% in the study conducted by Siddiqui et al.,21 with 40mcg of conventional vaccine, although the wide variety of vaccination regimens used (up to 14) and the limited use of a second cycle (21.5%) suggests that better immunological response may be achieved with more effective regimens. This strategy has already been tested in other studies, rescuing a large number of patients with a second cycle of conventional vaccine and a third cycle with adjuvanted vaccine.22 The second vaccination cycle in the PIBHE study rescued 19.5% of non-responders. Despite that the clinical practice guidelines are not totally clear in terms of vaccination and booster protocols, there is consensus on the need to double the conventional vaccine dose.23 Of note, there are vaccination regimens with 10mcg, these are dose used in children, and with 20mcg, as well as adjuvanted vaccine doses of 40 and 80mcg, which to date have never been tested in clinical trials or controlled tests.
The positive assessment of most nephrologists (78%) regarding the usefulness of this questionnaire suggests the need to develop similar strategies to perform self-assessment of patient management and to adopt an interventionist approach.
This is the first study in the world that has been designed to assess the level of valuation and follow-up of HBV(+) patients and the vaccination and immunisation of HBV(−) haemodialysis patients. It highlights the fall in prevalence of HBV in haemodialysis patients in Spain over 10 years and the wide range of vaccination regimens together with the low immunisation rates, with a potential for improvement with the development of better strategies. A multidisciplinary approach including nephrologists and hepatologists would be necessary, together with an update clinical practice guidelines in terms of HBV vaccination in haemodialysis patients.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: García Agudo R, Aoufi Rabih S, Barril Cuadrado G, Proy Vega B, Arias Arias Á, Herruzo Gallego JA, et al. Estudio multicéntrico español PIBHE: prevalencia e inmunización de la infección crónica por el virus de la hepatitis B en pacientes en hemodiálisis en España. Nefrologia. 2016;36:126–132.