Denosumab is a human monoclonal antibody (IgG2) that binds with great affinity and specificity to RANKL and blocks activation of RANK, its receptor, on the surface of osteoclasts and their precursors, thereby reducing their activity and causing a decrease in bone resorption of trabecular and cortical bone. It is used for the treatment of osteoporosis and is administered every 6 months.1,2 It is not necessary to adjust the dose in renal failure, but there is an increased risk of hypocalcaemia.1
We present the case of a 36-year-old man with chronic kidney disease due to stage 4 focal and segmental glomerulonephritis, who started with a nephrotic syndrome 3 years earlier. He had a poor clinical course, with sustained nephrotic syndrome and deterioration of renal function, despite receiving treatment with corticosteroids, cyclosporin, mycophenolate and rituximab, with clinical evolution as steroid-resistant nephrotic syndrome. He developed symptomatic secondary osteoporosis, with significant lumbar pain and bone loss documented by densitometry, and vertebral compression fractures. He was evaluated by Rheumatology, and it was decided to start treatment with denosumab 60mg. At that time, the patient was being treated with prednisone (10mg/day), furosemide, chlortalidone, spironolactone, rosuvastatin, allopurinol, omeprazole and paracetamol. In addition, he was receiving 1.2g of calcium carbonate with 800IU of cholecalciferol.
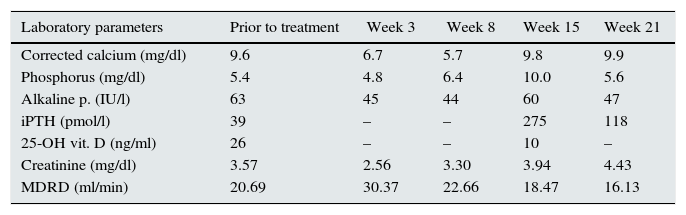
After 3 weeks of first dose of denosumab, the patient developed asymptomatic severe hypocalcaemia, which remained present and was even more severe at week 8. At that time, cholecalciferol was suspended and calcitriol (0.25mcg/48h) and calcium acetate (500mg/8h) were added. At week 15, serum calcium had returned to normal, but secondary hyperparathyroidism with hyperphosphoraemia related to his renal failure were observed. Calcitriol was suspended and a non-calcium chelator (sevelamer carbonate, 2.4g/day) was added. At 21 weeks biochemical parameters had improved (Table 1). The patient was asymptomatic throughout this period despite severe hypocalcaemia. Lumbar pain improved, but it was decided to suspend treatment with denosumab.
Laboratory data at baseline and over time.
| Laboratory parameters | Prior to treatment | Week 3 | Week 8 | Week 15 | Week 21 |
|---|---|---|---|---|---|
| Corrected calcium (mg/dl) | 9.6 | 6.7 | 5.7 | 9.8 | 9.9 |
| Phosphorus (mg/dl) | 5.4 | 4.8 | 6.4 | 10.0 | 5.6 |
| Alkaline p. (IU/l) | 63 | 45 | 44 | 60 | 47 |
| iPTH (pmol/l) | 39 | – | – | 275 | 118 |
| 25-OH vit. D (ng/ml) | 26 | – | – | 10 | – |
| Creatinine (mg/dl) | 3.57 | 2.56 | 3.30 | 3.94 | 4.43 |
| MDRD (ml/min) | 20.69 | 30.37 | 22.66 | 18.47 | 16.13 |
This was a young patient with stage 4 chronic kidney disease (CKD) who had osteoporosis following prolonged treatment with corticosteroids. Although he previously had normal levels of calcium, vitamin D, iPTH and alkaline phosphatase, he had probably also developed a bone mineral disorder associated with this (BMD-CKD).
BMD-CKD is more complex than osteoporosis. It includes all biochemical and skeletal abnormalities and extra-skeletal calcifications that occur as a result of abnormal mineral metabolism in CKD. Abnormalities in both remodelling and rate of remineralisation may occur, resulting in different types of bone disease (osteitis fibrosa, adynamic bone disease and osteomalacia).3,4 The use of an antiresorptive agent may have a different impact on the different types of bone disease, and could also have a different impact than in patients without CKD. Inhibition of osteoclast activity by denosumab could result in hungry bone syndrome.
There are only few studies on the use of denosumab in the treatment of osteoporosis that have included patients with advanced CKD, and these have included a limited number of patients.5–8 The FREEDOM study excluded patients with stage 5 CKD, and there were very few patients with stage 4 disease to assess whether the use of denosumab had significant benefit. All reports appear to indicate that there is an increased risk of hypocalcaemia, and that hypocalcaemia is severe, but the amount of information is limited and is hard to determine the severity of hypocalcemia with clear evidence, or to establish whether this effect is the same in the different types of BMD-CKD.
Notably, once hypocalcaemia has resolved, there is a marked increase in PTH, perhaps related to phosphorus retention due to renal failure which stimulates PTH synthesis and secretion.
More studies are needed to evaluate the safety of denosumab in CKD, especially in those with a GFR<30ml/min, and in the different forms of BMD-CKD. Until the available evidence of the therapeutic benefit of denosumab in this type of patient is more solid, it seems reasonable to assess its indication on a case-by-case basis and, if the treatment is indicated, there should be closer clinical follow-up, being alert for symptoms of hypocalcaemia and with more frequent laboratory testing than in patients with normal renal function.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Monge Rafael P, Arias M, Fernández-Fresnedo G. Hipocalcemia severa tras la administración de una dosis de denosumab en un paciente con insuficiencia renal avanzada. Nefrología. 2016;36:446–448.