Objetivo: Evaluar la cistatina C sérica y la microalbuminuria en la detección precoz de las alteraciones vasculares y renales. Material y método: La cistatina C sérica fue cuantificada en suero de un grupo de personas sanas y en un grupo de pacientes con enfermedad renal crónica para establecer un valor de cistatina C a partir del cual se pueda predecir un filtrado glomerular <60 ml/min/1.73 m2. Finalmente, la cistatina C sérica y la microalbuminuria fueron cuantificadas en pacientes con un incremento del riesgo de daño vascular y renal (hipertensión, diabetes e hiperlipemia). Resultados: Cuando la cistatina C sérica fue cuantificada en un grupo de riesgo, observamos cómo al aumentar los valores de cistatina C disminuían los valores del filtrado glomerular (p <0,05), que los valores de cistatina C se incrementaban al aumentar la edad de los pacientes (p <0,05) y cómo valores de cistatina C superiores a 0,95 mg/l no se observaron en pacientes con edad inferior a 50 años. En los pacientes del grupo de riesgo con un filtrado glomerular >90 ml/min/1,73 m2, la cistatina C sérica estaba elevada en un 27,6% con respecto a los valores obtenidos en personas sanas; existía microalbuminuria en un 20,3% y elevación de ambos parámetros en un 14,4%. Con valores de filtrado glomerular 60-90 ml/min/1,73 m2, la cistatina C estaba elevada en un 51,7%, la microalbuminuria en un 6,4% y ambos parámetros en un 23,8%. Conclusiones: Determinaciones de cistatina C sérica asociadas a la cuantificación de microalbuminuria en pacientes con riesgo pueden mejorar la detección del daño vascular y renal en estadios precoces. La cistatina C puede poner de manifiesto el daño vascular y renal precoz incluso en pacientes sin microalbuminuria.
Background: The aim of this study was to assess serum cystatin C and urinary albumin in the early detection of impairment in cardiovascular and renal function. Material ans methods: Cystatin C was quantified in sera from healthy people, moreover, cystatin C was quantified in a group of patients diagnosed with chronic kidney disease for predicting a measured glomerular filtration rate <60 ml/min/1.73 m2. Finally serum cystatin C and microalbuminuria were measured in patients with increasing of risk of impairment in cardiovascular and renal function (hypertension, diabetes and hyperlipidemia). Results: When the serum cystatin C was quantified in a group of risk, we observe as when being increased the cystatin C, the values of the glomerular filtration rate decreased (p <0.05), the cistatina values C were increased when increasing the age of the patients (p <0.05) and cystatin C values higher than 0.95 mg/l were not observed in patient smaller than 50 years old. In the group of risk, serum cystatin C was high regarding to the values obtained in healthy people in 27.6%, microalbuminuria in the 20.3% and both parameters were high in the 14.4% of patients with a glomerular filtration rate >90 ml/min/1.73 m2, while in patients with a glomerular filtration rate 60-90 ml/min/1.73 m2, cystatin C was high in the 51.7% and the microalbuminuria only in the 6.4%. Conclusions: Determinations of serum cystatin C associated to the quantification of urinary albumin in patients with cardiovascular risk can optimize the early detection of vascular and renal damage. Cystatin C can show vascular and renal damage in patients without urinary albumin.
INTRODUCTION
The relationship between systemic vascular disease and chronic kidney disease (CKD) has been obvious ever since it was shown that both dysfunctions have the same risk factors and mechanisms of progression.1 Hypertension, diabetes and hyperlipidaemia associated with the aging of the population have increased the prevalence of CKD.2 Thus, the presence of renal dysfunction in the elderly has been associated with increased cardiovascular risk and death. Most people with decreased glomerular filtration rate (GFR) are more likely to die due to cardiovascular diseases than to develop renal failure.3,4
Therefore, early-stage renal damage would reveal vascular damage. Early-stage renal damage may be revealed by a reduction in GFR.
The GFR may be estimated from the serum creatinine levels using equations such as the MDRD4,5 the equation that most scientific societies have chosen for measuring renal function.6,7
According to the criteria established by the 2002 KDOKI guidelines (National Kidney Foundation), CKD is defined by the kidney damage (determined either directly by biopsy, or indirectly by the presence of microalbuminuria, proteinuria, abnormal urinary sediment or abnormal findings in imaging studies) or by a GFR<60ml/min/1.73m2 for at least three months.8
Increased urinary albumin excretion is an established risk for mortality, cardiovascular disorder and adverse outcomes, both in the general population and in patients with hypertension and diabetes.
Increased albumin in urine is also a marker of diffuse vascular damage, systemic inflammation, renin-angiotensin system activation and glomerular disorders or abnormal tubular function.9,10
Cystatin C has been identified as a promising new marker for early detection of early renal damage, and is more sensitive than creatinine.11,12 Cystatin C is produced by all nucleated cells at a constant level,13,14 is freely filtered by the glomerulus and almost completely reabsorbed and degraded, but not secreted, by the proximal tubular cells. However, its production is not affected by age, sex and muscle mass.15-17 Cystatin C is strongly correlated with serial measurements of GFR by intravenous infusion of an exogenous marker (iothalamate).18,19 Therapy with high doses of corticosteroids, thyroid dysfunction,20 neoplasms21 and human immunodeficiency virus (HIV) infection22 may change cystatin C values.
The aim of this study was to assess early vascular and renal damage in patients with cardiovascular risk factors, such as essential hypertension, diabetes and hyperlipidaemia, but with GFR>60ml/min/1.73m2, normal urea and creatinine levels for their sex and age, and with normal urinary sediment, through the quantification of serum cystatin C and urinary albumin.
MATERIAL AND METHOD
Patients
For this cross-sectional descriptive study, we selected the sera of 456 patients between the ages of 19 and 89 years. Patients were from the Hospital Universitario Infanta Cristina of Badajoz and its area. The selection was based on the patient medical history and sought to establish three groups:
Control group (C). We selected sera from 61 healthy individuals with stage 1 GFR, without microalbuminuria or known disease.
The chronic kidney disease (CKD) group was made up of sera from 71 patients diagnosed with CKD, according to the criteria established by the 2002 KDOKI guidelines (National Kidney Foundation). All patients came from our hospital's nephrology department.
The risk group (RG) was made up of sera from 324 patients with risk for CKD, but with urea and serum creatinine levels within normal ranges for their sex and age, and with a GFR>60ml/min/1.73m2. Within this group we found 23 patients with essential hypertension (7.09%), 60 with essential hypertension and hyperlipidaemia (18.51%), 47 with diabetes (14.50%), 111 with diabetes and hyperlipidaemia (34.25%), 48 with hyperlipidaemia (14.81%) and 35 patients with essential hypertension, diabetes and hyperlipidaemia (10.80%).
Patient exclusion criteria included diseases that may cause falsely high results for cystatin C, such as hypothyroidism, HIV and neoplasms.
Selection of variables
Diabetes, hypertension, hyperlipidaemia and GFR>60ml/min/1.73m2 were selected as defining variables for our risk group.
Diabetes was defined as a medical history of diabetes, the use of antihyperglycemic drugs or insulin, or fasting glucose levels ≥6.99mmol/l. Hypertension was defined by the systolic blood pressure range ≥140/190mm Hg and/or antihypertensive treatment. Hyperlipidaemia was defined as cholesterol ≥6.5mmol/l, high-density lipoprotein cholesterol (HDL-C) <1.55mmol/l, triglycerides >3.89mmol/l, low-density lipoprotein cholesterol (LDL-C) >2.59mmol/l or the use of lipid-lowering medication.
GFR values were divided into five stages (1: >90; 2: 60-89; 3: 30-59; 4: 15-29, and 5: GFR<15ml/min/1.73m2).
Cystatin C was considered high according to the recommendations of the manufacturer, with a reference range of 0.53mg/l to 0.95mg/l for individuals with no history of kidney disease.18
Normoalbuminuria was defined as an albumin/creatinine ratio (A/C) <2.5mg/mmol in males and an A/C ratio <3.5mg/mmol in females. Microalbuminuria was defined as an A/C ratio >2.5mg/mmol in males and >3.5mg/mmol in females.8
Method
Blood samples were taken between 08.00 and 09.00 hours after 12 hours of fasting. Serum was separated by centrifugation, and routine analysis was performed that same morning. Serum cystatin C and urinary albumin (first morning urine) were kept at 4ºC and analysed at 24 hours.
Cystatin C concentrations were quantified by immunonephelometry assay in a BN ProSpec® autoanalyser (Siemens Health Care Diagnostic, Deerfield, USA). Albumin was measured by immunoturbidimetric assay in an ADVIA® 2400 Chemistry System autoanalyser (Siemens Health Care Diagnostic, Deerfield, USA).
Serum creatinine (for calculating GFR) and urinary creatinine (for calculating the microalbumin/creatinine ratio) were measured using the Jaffe method in an ADVIA® 2400 Chemistry System autoanalyser (Siemens Health Care Diagnostic, Deerfield, USA).
The MDRD4 equation was used to estimate GFR.7
The study was approved by the ethics committee of Hospital Universitario Infanta Cristina of Badajoz.
Statistical analysis
Statistical analysis was performed using Microsoft Excel® (Microsoft Office software) and the SPSS statistical program for Windows.
Descriptive analysis was performed using the mean and standard deviation (SD) as central and dispersion measures, after checking the normality of the groups with the Kolmogorov-Smirnov test.
Reference intervals were calculated using the mean ±2 SD.
Correlations between variables were evaluated using the Pearson’s correlation coefficient.
Comparison of means between variables with two categories was performed depending on the nature of the variables using the chi-square test and Student's t-test. For those variables with more than two categories, the analysis of variance (ANOVA) test was used.
We used an ROC (receiver-operating characteristic) curve analysis to establish a cutoff point and determine the ability of cystatin C to predict a GFR<60ml/min/1.73m2 (gold standard).
RESULTS
Patients from groups C, CKD and RG were assessed for age, cystatin C values and GFR (eGFR), as shown in Table 1. A statistically significant correlation was found (P<.05) between cystatin C and GFR values from all patients in the three groups (R2=0.931), and between cystatin C and age for the RG group (R2=0.460).
The cystatin C values among the three groups were also compared, and statistically significant differences were obtained (P<.05) between the C group and the CKD group, and between the RG and CKD group. We also calculated the cutoff point for diagnosing CKD (GFR<60ml/min/1.73m2) for all patients in our study, with a cystatin C value of 0.95mg/l for 92% sensitivity and 82.2% specificity.
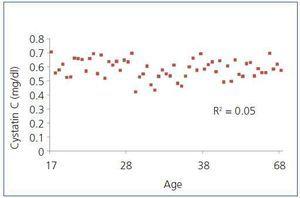
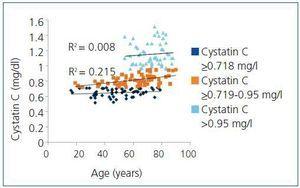
With regard to age, it was common for patients in the RG that cystatin C values increased with age, a relationship that was statistically significant (R2=0.460; P<.05). This was contrary to what happened in the C group, where we observed that these values remained constant with age (R2=0.05; P>.05) (Figure 1). When we calculated the correlation between cystatin C and age in this group, we observed that for patients with cystatin C values <0.718mg/l, values between 0.718mg/l and 0.95mg/l and values above 0.95mg/l, the results were R2=0.283 (P<.05), R2=0.395 (P<.05) and R2=0.093 (P>.05). There were no statistically significant correlations in patients with cystatin C values >0.95mg/l, or statistically significant differences between patient age among these three cystatin C values.
In the RG, we also observed that cystatin C values were above 0.95mg/l (cutoff point for diagnosing CKD [GFR<60ml/min/1.73m2]) only in individuals over 50 years of age. Meanwhile, cystatin C values <0.718mg/l (high value for cystatin C in C group) and between 0.718mg/l and 0.95mg/l were detected in all age ranges, with a starting age of approximately 20 years (Figure 2).
Regarding the GFR in the RG, we observed that cystatin C values increased as GFR decreased (Figure 3), a correlation that was statistically significant (R2=0.502; P<.05). Therefore, patients in this group with GFR>90ml/min/1.73m2 (152 patients) had a mean cystatin C value of 0.700mg/l (0.664-0.736). The remaining 172 patients in this group with GFR between 60 and 89ml/min/1.73m2 had a mean cystatin C value of 0.824mg/l (0.524-1.124), with statistically significant differences (P<.05) between cystatin C values of patients with GFR in stages 1 and 2.
With regard to the determination of urinary albumin in the RG, we found that 40.43% of patients had isolated elevation of cystatin C, 12.96% had isolated microalbuminuria and 19.44% had both elevation of cystatin C and microalbuminuria. There were no statistically significant differences between cystatin C values of patients with normoalbuminuria (cystatin C=0.850mg/l [0.386-1.314]) and microalbuminuria (cystatin C=0.888mg/l [0.458-1.318]).
After reassessing this group based on GFR (Table 2), we observed that among the patients with GFR 1, the percentage of cases that had high cystatin C with normoalbuminuria (27.6%) was slightly higher than the percentage of patients that had isolated microalbuminuria, with no elevation of cystatin C (20.3%), or elevation of both parameters (14.4%). However, in patients with GFR 2, elevation of cystatin C without microalbuminuria (51.7%) was much higher than the patients who had microalbuminuria, both isolated (i.e. with no elevation of cystatin C [6.4%]) and with elevation of both parameters (23.8%). There was no statistically significant relationship, however, in any of the cases (P>.05).
DISCUSSION
A higher life expectancy and evolution of treatments have increased the prevalence of a series of diseases including hypertension, diabetes and hyperlipidaemia, which, in turn, have increased the prevalence of CKD and cardiovascular diseases.2
Several studies have demonstrated the usefulness of cystatin C as an ideal marker of renal function11 and the relationship that exists between cystatin C values and increased cardiovascular and renal risk in various population groups and associated diseases.23,24 In our study, we have attempted to establish the importance of measuring cystatin C values along with traditionally studied parameters, such as microalbuminuria, in a group of patients with clearly established cardiovascular risk factors (hypertension, diabetes and hyperlipidaemia). These patients, however, were in very early stages of renal involvement and presented stage 1 or 2 renal function (GFR>60ml/min/1.73m2), normal urea and creatinine levels for their sex and age, and had normal urinary sediment.
Various authors have reported increased cystatin C values with advancing age.26,27 However, the populations of these studies were heterogeneous groups of individuals of different ages, and included both healthy individuals and those with risk factors.
We have considered healthy individuals and those with risk factors as different groups, observing how the cystatin C values in the C group did not increase with age, as reported by some authors.15-17 However, in the RG, we observed quite the opposite: cystatin C values increasing with age, as published by other authors.24 These authors found a significant association between high cystatin C values and various risk factors (increasing age, systolic blood pressure, haemoglobin A1c and triglycerides, and decreasing HDL-C and microalbuminuria, among others).
Therefore, within this RG, for patients with cystatin C values <0.718mg/l, aged 20 years to 78 years and a GFR between 65 and 146ml/min/1.732, there was a statistically significant correlation between cystatin C values and age (R2=0.283; P<.05). The same occurs with patients with cystatin C values from 0.718 to 0.95mg/l, aged 19 years to 89 years and a GFR from 64 to 123ml/min/1.73m2, with an R2=0.395 (P<.05). From these data we can deduce that this increase in cystatin C in patients within these two age ranges is due to factors that cause decreased an age-dependent reduction in GFR. However, one must take into consideration that these patients have risk factors (hypertension, diabetes and hyperlipidaemia) that increase the prevalence of CKD. Moreover, we observe that this trend is broken when cystatin C values are >0.95mg/l, and there is no statistically significant correlation (P>.05) between age and cystatin C values in this group (R2=0.093) for an age range from 51 to 86 years and GFR of 60 to 99ml/min/1.73m2. This suggests that increased cystatin C in these patients is dependent on the time of evolution of their disease and control, more so than on the age of the patient, since the age-dependent decrease in GFR (approximately 12ml/min per decade) begins at age 50.27 If we knew the exact time of evolution of risk factors, this could indicate that patients over 50 years of age would be those with a high risk for developing vascular disease and CKD due to a poorer evolution of their disease over the years. This would then justify the readings obtained for cystatin C due to poor control of their disease. Patients with cystatin C levels below 0.718mg/l are therefore well controlled, while those with cystatin C levels between 0.718and 0.95mg/l deserve special attention, since they are candidates for presenting a risk of early vascular and renal damage.
Microalbuminuria is widely studied renal dysfunction marker, since its presence is an established risk for mortality, cardiovascular disorders and adverse outcomes, both in the general population and in patients with hypertension and diabetes.
Some authors have established a relationship between increased cystatin C and subsequent appearance of microalbuminuria.25 When we determined urinary albumin in the RG, we found no correlation between the cystatin C values and urinary albumin, or statistically significant differences between cystatin C values in patients with normoalbuminuria and microalbuminuria.
However, according to the results, quantification of cystatin C in patients with GFR 1 and 2 makes it clear that patients show early renal and vascular damage with no microalbuminuria, and that they, as well as patients with microalbuminuria, may benefit from proper preventive and therapeutic measures. The serial measurement of both parameters would allow us to detect a greater number of patients in early stages of CKD and would indicate how the disease evolves in these patients, allowing us to adopt early measures to control the disease.
Study limitations
Since this is a cross-sectional descriptive study, the main limitation of the study is the inability to detect renal and vascular damage through reference methods, such as the use of radioactive isotopes for measuring renal function or intima-media thickness to detect vascular damage. We were also unable to determine the exact time of evolution of the various risk factors in the RG.
Table 1. Characteristics of the study population
Table 2. Patients with microalbuminuria and/or high cystatin C in the risk group
Figure 1. Relationship between cystatin C and age in the control group
Figure 2. Classification of risk group patients depending on cystatin C values and age
Figure 3. Figure 3 - Relationship between cystatin C and glomerular filtration rate in the risk group