Antecedentes: La frecuencia de tumores en pacientes con trasplante renal (TR) está aumentando. El carcinoma de células renales (CCR) en riñones nativos es uno de los más frecuentes y su evolución puede ser más agresiva que en la población general. Objetivo: Evaluar la incidencia y pronóstico del CCR en los pacientes sometidoa a trasplante renal de nuestra unidad. Métodos: Desde enero de 1997 hasta diciembre de 2009 se realizaron en nuestro hospital 683 TR. Para la detección precoz de esta patología, en todos los pacientes sometidos a trasplante realizamos una ecografía abdominal anual de forma sistemática. Los casos sospechosos se confirmaron con tomografía computarizada (TC) antes de proceder a la nefrectomía. Resultados: Por sospecha de CCR se realizaron 14 nefrectomías radicales en 12 pacientes. En 11 nefrectomías (correspondientes a 9 pacientes), el diagnóstico anatomopatológico fue de CCR (incidencia del 1,5%). Todos fueron diagnosticados en estadio T1N0M0. En los tres pacientes restantes, en los que no se detectó tumor, el diagnóstico fue de quiste renal complicado en el contexto de poliquistosis hepatorrenal. En los pacientes con CCR, el tiempo en diálisis antes del diagnóstico fue de 36,7 ± 24,3 meses y el intervalo entre el TR y el diagnóstico fue de 39 ± 25,8 meses. Tras un seguimiento medio de 58,6 ± 38,6 meses, la evolución en todos ha sido excelente, sin recurrencia del tumor. Conclusiones: La realización de ecografías anuales permite un diagnóstico precoz del CCR. El tratamiento de esta patología en estadios iniciales se asocia con un excelente pronóstico en los pacientes con TR.
Background: The frecuency of malignancies in renal transplant (RT) patients is increasing. Renal cell carcinoma (RCC) of native kidneys is one of the most frequent and its outcome can be more aggressive than in general population. Objetive: To evaluate the incidence and prognosis of RCC in renal transplant patients followed in our transplantation unit. Methods: Between January 1997 and December 2009, 683 patients underwent kidney transplant at our hospital. Ultrasonography of the native kidneys was annually performed in all renal transplant patients. When suspect solid masses were found at ultrasonography, patients underwent computed tomography. If the suspicion was confirmed, nephrectomy was performed. Results: 14 radical nephrectomies were performed in 12 patients due to suspect CCR. In 11 nephrectomies (corresponding to 9 patients), anatomopathologic diagnosis was CCR (incidence 1.5%). Histologic stage was T1N0M0 in all cases. In the other 3 RT, the diagnosis was complicated renal cyst. Those patients without carcinoma had polycystic kidney disease. The time on dialysis before CCR diagnosis was 36.7 ± 24.3 months and the interval between RT and diagnosis was 39 ± 25.8 months. After a mean follow-up of 58.6 ± 38.6 months, the outcome of all cases has been excellent, without tumor recurrence. Conclusions: Annual renal ultrasonography plays a key role in the early diagnosis of CRR. The early treatment of this pathology is associated with an excellent prognosis in RT patients.
INTRODUCTION
In recent years, patient and renal graft survival has increased thanks to the introduction of new immunosuppressive drugs and better management of short and long-term complications.1 However, this increased survival, as well as the progressive increase in the average age of patients on the waiting list for kidney transplantation (KT), have increased the incidence of cancer in this population.1,2
One of the tumours whose incidence has increased the most is renal cell carcinoma (RCC) in native kidneys.3 Its emergence has been linked to various risk factors including immunosuppressive therapy, age, prior history of RCC and acquired cystic kidney disease (ACKD).3-6 Numerous studies confirm that tumour size at diagnosis has a significant impact on prognosis, which is much better for small tumours.5
Abdominal ultrasound is a simple and useful diagnostic method for early detection of these tumours. However, there is no consensus on the optimal frequency with which these examinations should be performed.4-7
The aim of this study was to evaluate the incidence and prognosis of RCC in KT performed in our department from January 1997 to December 2009, monitoring all patients for the development of these tumours using annual ultrasounds.
MATERIAL AND METHOD
Since 1997, all patients who have undergone KT in our unit have been regularly monitored using a neoplasm detection protocol. As the routine detection test for RCC in native kidneys, we performed annual systematic abdominal ultrasounds in all patients undergoing KT. When the ultrasound showed lesions suspicious of RCC, we completed an abdominal computed tomography (CT) study. If the suspicion was confirmed, we proceeded to perform a nephrectomy and a histology study on the tumour. We analysed the results of 683 KT performed in our hospital from January 1997 to December 2009.
We collected demographic and clinical data from the patients with RCC, including age, sex, primary kidney disease, dialysis modality prior to KT, dialysis duration, number of KT, immunosuppressive treatment, time from the KT to the diagnosis of RCC and renal function at the time of the nephrectomy.
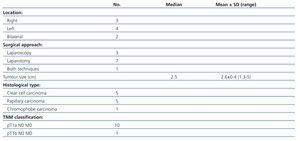
Regarding the diagnosis of RCC, we analysed the surgical approach and the location, size, histological type and stage of the tumour. The latter was stratified according to the international TNM classification.8 For clear cell carcinomas, the nuclear grade was classified according to the criteria proposed by Fuhrman.9
The coexistence of ACKD was also assessed, as well as the appearance of tumours in other locations and whether changes were made to the immunosuppressive therapy after the RCC diagnosis. Finally, we recorded patient evolution after nephrectomy, survival and renal function during follow-up, and whether there was tumour recurrence.
The results are expressed as median and mean ± standard deviation for quantitative variables, and as absolute and/or relative frequency for qualitative variables.
RESULTS
Due to suspicion of RCC, 12 patients underwent 14 radical nephrectomies. In three patients, each one undergoing a unilateral nephrectomy, there were no tumours. These three patients had hepatorenal polycystic disease as their primary kidney disease. As with all cases, a pre-nephrectomy CT was performed in these three cases, with the diagnosis of cystic renal mass (Bosniak category III). Additionally, Doppler ultrasound with echo-enhancer (using sulphur hexafluoride as an IV contrast) was performed in the three cases. In two cases, the test indicated a neoplasm, but it was doubtful for the third one. Finally, nephrectomy was performed in the three cases, and the pathological diagnosis was complicated renal cysts.
In the nine remaining KT patients (seven unilateral nephrectomies and two bilateral nephrectomies), 11 cases of RCC were detected (five clear cell carcinomas, five papillary carcinomas and one chromophobe cell carcinoma). In one of the two bilateral nephrectomy patients, we performed a bilateral nephrectomy for bilateral RCC in the same surgical act. The other patient underwent a second nephrectomy a year after the first nephrectomy due to appearance of another RCC in the contralateral kidney.
In our series, the incidence of RCC in native kidneys was 1.5%. Demographic characteristics of RCC patients are shown in Table 1. At the time of tumour diagnosis, all patients except one had been on renal replacement therapy with dialysis and transplantation more than six years. There were no cases of analgesic nephropathy and only one patient had ACKD.
All patients received immunosuppressive therapy with mycophenolate mofetil and calcineurin inhibitors from KT. Only one patient received treatment with cyclosporine A, with the rest on tacrolimus. Three patients received induction with anti-CD25 antibodies. None of the patients had a history of acute rejection episodes. All patients were asymptomatic at the time of diagnosis, which was an accidental finding in the routine abdominal ultrasound.
Tumour characteristics are shown in Table 2. Radical nephrectomy was performed in all cases: seven by laparotomy (the earliest cases when laparoscopy had not yet been incorporated into our hospital) and three by laparoscopic approach. Conversion to open surgery was necessary for one patient due to complications in the laparoscopic surgery. The average tumour size was 2.6±0.45cm (range: 1.3 to 5). All tumours, except one, were less than 3.5cm in diameter. All cases of RCC were diagnosed in stage T1N0M0. The Fuhrman nuclear grade was II in the five clear cell carcinomas.
Changes were made to all patients’ immunosuppressive therapy. In five patients, the calcineurin inhibitor was converted to an mTOR inhibitor, with good tolerance in all cases, except for one case where the calcineurin inhibitor had to be reintroduced due to uncontrollable oedema and proteinuria. In the remaining four cases, the use of mTOR inhibitors was contraindicated (renal failure and/or proteinuria). Therefore, immunosuppressive therapy was reduced, decreasing the dose of calcineurin inhibitor and/or mycophenolate mofetil, or discontinuing the latter.
After an average follow-up of 58.6±38.6 months (range: 15 to 110), all patients had excellent evolution, with 100% patient survival and no tumour recurrence. Three patients were later diagnosed with new tumours in other locations (two were diagnosed with basal cell skin carcinoma and one was diagnosed with a vesical tumour and a basal cell skin carcinoma).
Two patients required new inclusion in dialysis for chronic graft nephropathy, which had been diagnosed before the RCC finding. Re-inclusion in dialysis occurred at 44 and 47 months from the diagnosis of the RCC, respectively. For the other patients, renal function was optimal, with an average serum creatinine of 1.12±0.3mg/dl (range: 0.7 to 1.5) at the end of follow-up.
DISCUSSION
The increased survival of patients undergoing KT and the progressive increase in average age of patients on waiting lists for KT, has made long-term complications, such as cardiovascular disease and cancer, increasingly important in this population.2 Although cardiovascular disease is still the main cause of death for these patients, the incidence of cancer has significantly increased in recent years, especially in older recipients.1
In general, the risk of developing neoplasms increases for patients with chronic kidney disease. The main associated risk factors include age, previous exposure to oncogenic agents (tobacco, alcohol, sun exposure), genetic predisposition, as well as the aetiology of kidney disease, time in dialysis and uraemia-related immune dysfunction.2-6 Nevertheless, the incidence of cancer is even greater after KT, mainly due to the role of immunosuppression, along with other factors such as reactivation of viruses with oncogenic potential, age at the time of transplantation and time elapsed since transplatation.1-5 Moreover, cancers in patients undergoing KT generally have a more aggressive nature, with more rapid progression and a poorer response to treatment.3,5
The most frequent tumours in the population undergoing KT are skin, genitourinary and lymphoproliferative neoplasms. Kasiske et al found that the incidence of non-melanocytic skin tumours, Kaposi's sarcoma and non-Hodgkin lymphoma was 20 times higher than in the general population.4 In recent years, an increase has also been observed in the appearance of native kidneys RCC in patients undergoing KT, for whom it is 10 to 100 times more frequent than in the general population.3 Its incidence varies significantly, between 0.3% and 4.8% depending on the series, probably because there is no agreement on how to perform diagnostic screening for this disease.5
Several risk factors have been associated with the appearance of RCC in the KT population, which include immunosuppressive therapy, time in dialysis, age, prior history of RCC, analgesic abuse and ACKD.3-6,10-13 In our experience, time in renal replacement therapy was prolonged in almost all cases, with a median of 31 months on dialysis before KT, and 52 months from the KT to the development of the RCC. Only one of our patients had ACKD, and it was this patient who was the longest on dialysis (82 months) before the diagnosis of RCC. This condition is considered an independent risk factor for RCC, although its absence does not rule it out,14 as we have shown in our series.
The course of RCC in KT is more aggressive than in the general population, with a less favourable prognosis and poorer response to treatment.5,15 Moreover, numerous studies confirm that tumour size at diagnosis has a significant impact on the evolution of the disease, and that the prognosis is much better for tumours smaller than 3cm in diameter.5 We also know that treatment in the initial stages of this disease is associated with excellent evolution, and consequently the importance of early diagnosis of this condition, especially in KT patients.5,16,17
Abdominal ultrasound is a simple and non-invasive diagnostic method, which is useful in detecting RCC in the native kidney of KT patients. However, the guidelines of American and European societies of nephrology and urology have not yet established how often these examinations should be performed for the early detection of RCC in native kidneys in KT.4-7 Some groups recommend performing annual ultrasound studies in asymptomatic patients, and every six months in high-risk patients.5,15 In contrast, other authors perform them every three years.3 In our experience, all patients were asymptomatic at the time of diagnosis, which was an accidental finding in the routine screening that we carry out in our unit. Annual ultrasounds allowed us to diagnose all cases very early.
In our study, as reported in other series,10,18 the three cases of false positive were patients with hepatorenal polycystic disease with complicated cysts (Bosniak III). This highlights the difficulty in diagnosing RCC in this disease, given that those two lesions are not always easy to distinguish. According to the Bosniak classification for renal cystic masses, stage III is at high risk of being malignant, as was the case in our patients.19 Moreover, the incidence of RCC in polycystic patients with complicated cysts may be high (up to 50%, according to Schwarz et al).20 Therefore, in patients with Bosniak III renal cysts, nephrectomy is recommended, as we eventually did in our cases.20,21
Among the most important prognostic factors for CCR, in addition to tumour size, are staging (TNM classification) and the Fuhrman nuclear grade.9,10 Thus, survival at five years clearly varies according to the TNM stage, with a rate of 90%-100% in stage I (T1N0M0), and less than 10% in stage IV, if distant metastases are present.17,22 According to the Fuhrman nuclear grade, RCC are divided into low (I and II) and high (III and IV) grades. In our series, the screening by ultrasound has meant that, at the time of diagnosis, the tumours were small, all in stage T1N0M0 with a low Fuhrman nuclear grade. This has definitely influenced the excellent evolution observed.
One patient in our series, one year after the first nephrectomy for RCC, developed another RCC in the contralateral kidney, and three other patients were diagnosed after the RCC with tumours in other locations. This suggests that these patients had a greater predisposition to developing cancer, as has been observed in other groups.11 In those patients, therefore, it is important to be especially careful during follow-up and vigilant for possible new neoplasms.
The link between immunosuppressive therapy and the greater risk of tumours in patients undergoing transplantation is well known.23 Calcineurin inhibitors have been associated, in general, with an increase in the incidence of cancer. One study found that this incidence is greater with tacrolimus than with cyclosporine.24 In our case, almost all patients were treated with tacrolimus, as it was the most common immunosuppressive therapy during the time period when this study was conducted. In contrast, proliferation signal inhibitors have been shown to play an antitumor role. Several studies have reported that mTOR inhibitors may be beneficial in the treatment of Kaposi's sarcoma, recurring skin cancer, renal cell carcinomas and other solid tumours.25-29 In all of our patients, we modified the immunosuppressive therapy, converting from calcineurin inhibitors to mTOR inhibitors where possible, and reducing immunosuppression for all patients. This, along with early surgical treatment of RCC, is likely to have contributed to our good results.
The evolution of all our patients was excellent, with a 100% survival, and without recurrence of the disease during follow-up. Although the sample size was small, our experience shows that, despite the greater incidence of RCC in KT patients, compared to the general population, the prognosis for this disease is excellent if treatment is performed in the initial stages. Therefore, annual abdominal ultrasounds should be performed in KT patients as an early detection method for RCC.
Table 1. Patient characteristics
Table 2. Characteristics of renal cell carcinomas