To the Editor,
Current advances in nephrology and similar advances in other areas of medical knowledge mean that nephrologists must develop technical skills that are not provided by traditional training in nephrology. We present a case that illustrates that fact.
The patient in question is an 83 year old male in a conventional haemodialysis (HD) programme with chronic kidney disease secondary to diabetic nephropathy. He had a history of type 2 diabetes mellitus with various diabetes-related complications, arterial hypertension and atypical chest pain with no evidence of ischaemic heart disease.
The patient started HD via a tunnelled catheter in February 2010, with good haemodynamic tolerance and adaptation. A left humeral-cephalic arteriovenous fistula (AVF) was created one month later. Following a 30-day maturation period, we began venipuncture in the AVF and observed suboptimal maturation, difficult anatomical interpretation, venous collapse, ´frequent extravasations and impossibility of reaching a blood flow (Qb) greater than 250ml/min.
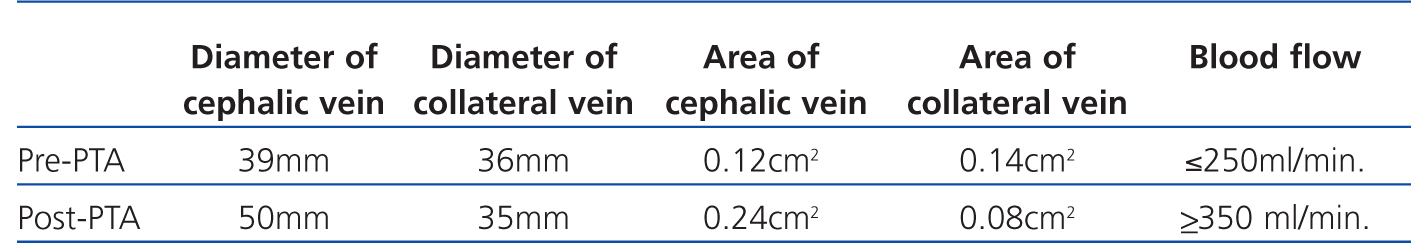
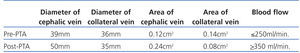
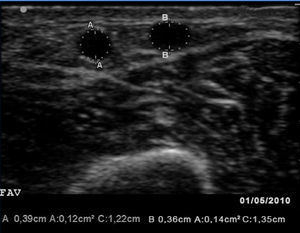
Given these findings, we examined the vascular access (VA) with a portable vascular ultrasound machine (EcoAVP) in the HD room (Figure 1) and observed no stenosis in the arteriovenous fistula and a dual venous system with a collateral vessel branching off 3cm from the arterial anastomosis with a thickness similar to that of the two veins (diameter: 0.39cm vs 0.36cm; area: 0.12 vs 0.14cm2). We found 2 stenoses in the proximal part of the cephalic vein.
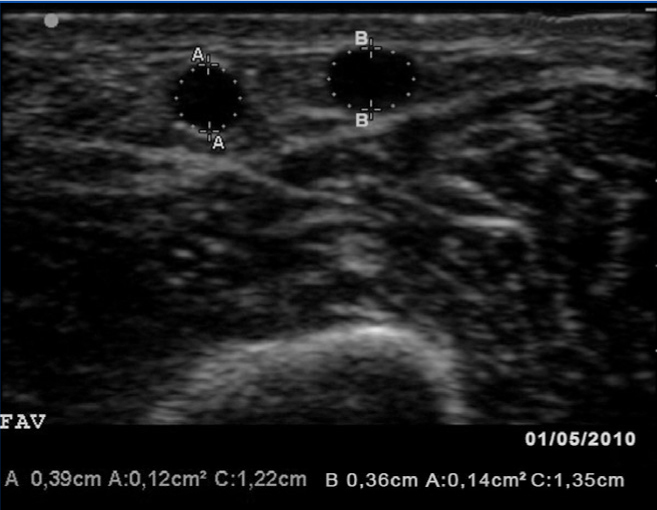
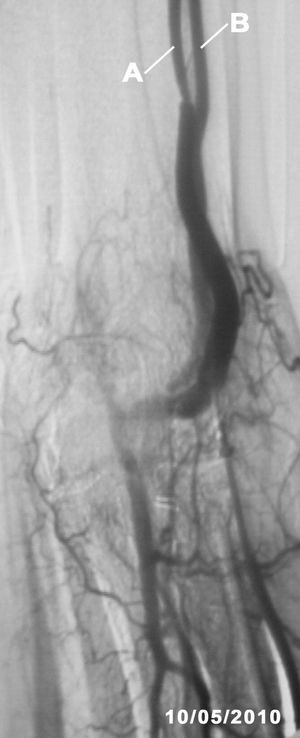
The fistulography (Figure 2) confirmed the ultrasound findings, a haemodynamically significant (80%) stenosis at 10cm from the arteriovenous fistula and another smaller one in the proximal third of the cephalic vein. Percutaneous angioplasty was performed on the 2 stenoses with good angiographic results. The identified collateral vessel was not treated in any way.
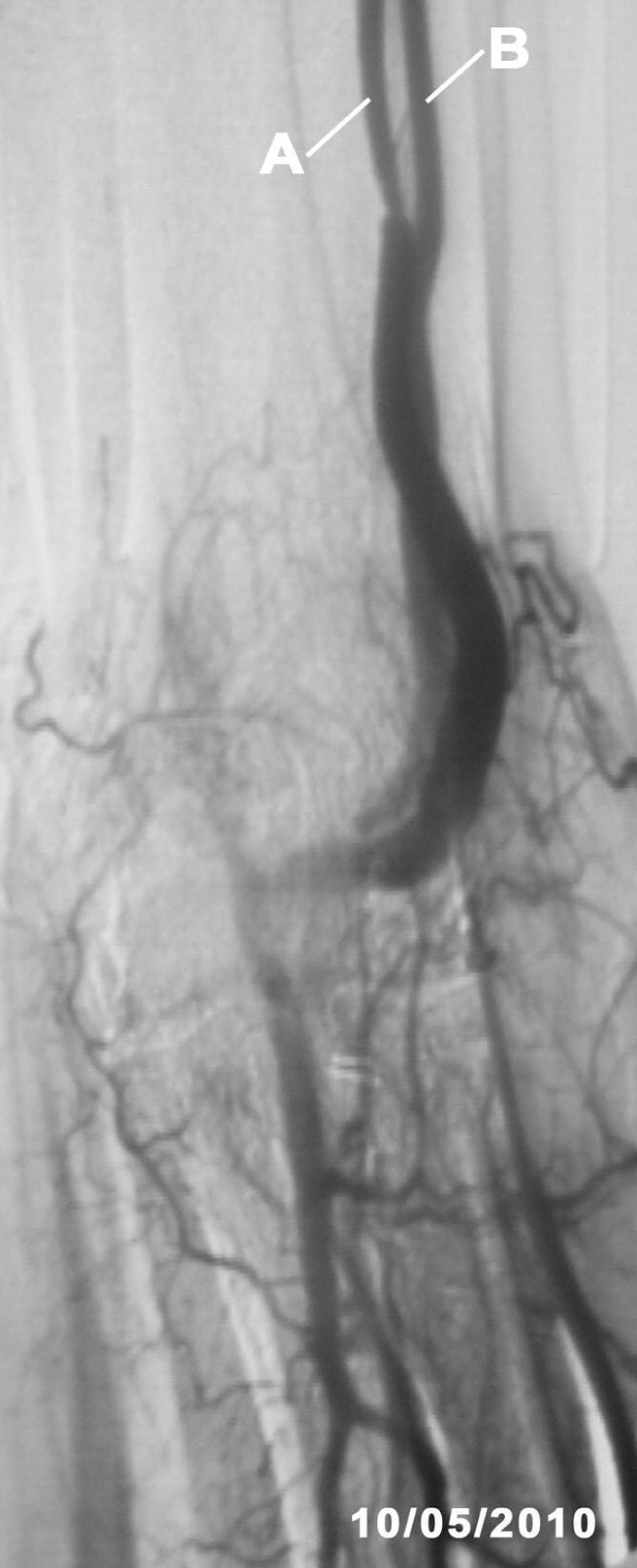
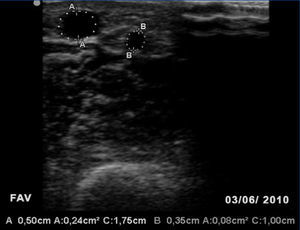
One month later, the AVF had progressed well, allowing for cannulation with no extravasations and an acceptable Qb rate. A second image from the EcoAVP (Figure 3) confirmed the increase in the diameter and the cross-sectional area of the main vein (diameter: 0.5cm, area: 0.24cm2) with a decrease in the size of the collateral vessel (diameter: 0.35, area: 0.08cm2).
One year later, the AVF was functioning properly, with a Qb of 350ml/min and a normal venous pressure of 140mmHg.
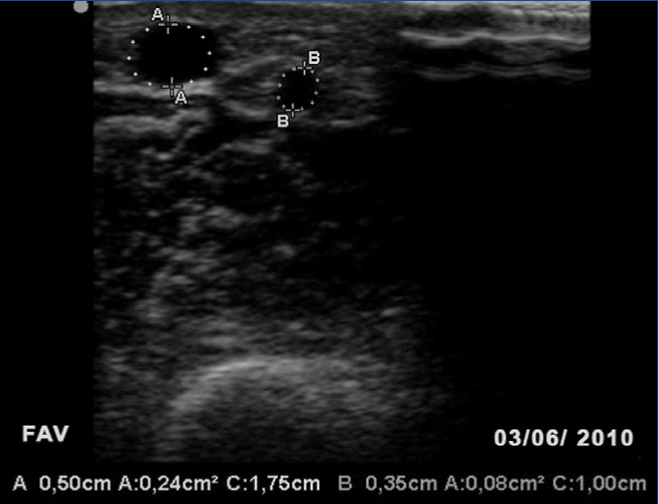
Table 1 shows the changes in some clinical parameters and ultrasound images taken after the treatment with percutaneous angioplasty.
The use of an EcoAVP is not common in daily practice. However, it is very useful for approaching, monitoring, and diagnosing AVF complications.1 Ultrasound provides both morphological and functional information in a fast, reliable and non-invasive way, which helps us determine whether percutaneous or surgical treatment is necessary.2 The EcoAVP enables us to combine B-mode imaging, which estimates vein volume, the presence of haematomas, parietal calcifications, intraluminal thrombi, collateral vessels and stenosis, with the Colour Doppler mode, which estimates blood flow, peak systolic velocity, the presence of turbulences, and the shape of pulse waves with the corresponding resistance indices.3 Ultrasound results must always be interpreted in conjunction with clinical findings.3
A broader view of the nephrologists’ participation in decision-making would include using ultrasound for arterial and venous mapping, which has been proven to increase success in surgical interventions,4,5,6,7 and estimated venous elastography as a tool that may predict AVF success (limited evidence at present).5
At present, guidelines do not set strict criteria for periodical ultrasound assessments of VA or recommend a time to initiate ultrasound monitoring. In some studies, the complications involved in VA failure, which can be detected with a EcoAVP, are present in AVF that still function normally.8 On the other hand, early dysfunction and primary failure in radiocephalic AVF and the frequent delayed maturation in diabetic patients leads us to recommend using a EcoAVP as a monitoring device for all patients on dialysis.4 Considering the increased mean age of patients in dialysis units and data on the high number of complications at any level and any type of VA in elderly patients,4,9 we can state that training in ultrasound examinations should be included in the nephrological curriculum. Active participation of nephrologists in the diagnosis and treatment of VA complications may reduce the number and duration of hospital stays associated with such problems, reduce the use of venous catheters, shorten waiting times for having an AVF, reduce costs derived from diagnostic and therapeutic procedures, and optimise prevention of complications in general.10
Despite a certain amount of dependence on specialties such as vascular surgery or interventional radiology in this field, the nephrologist is ultimately responsible for ensuring that the VA works correctly. This responsibility requires strict monitoring and early treatment of VA complications in a multidisciplinary area that encounters frequent administrative obstacles. Proper training in ultrasound examinations will enable professionals to make better treatment decisions in situations in which success depends upon swift action.
Table 1. Changes in certain study parameters following percutaneous transluminal angioplasty of the arteriovenous fistula
Figure 1. First B-mode ultrasound image of the vascular access in which we see two veins of similar size
Figure 2. Fistulography image taken after percutaneous angioplasty to both stenoses
Figure 3. Second B-mode ultrasound of the arteriovenous fistula showing an increase in cephalic vein size and decrease in the width of the collateral vessel