Paracellular channels occurring in tight junctions play a major role in transepithelial ionic flows. This pathway includes a high number of proteins, such as claudins. Within renal epithelium, claudins result in an ionic selectivity in tight junctions. Ascending thick limb of loop of Henle (ATLH) is the most important segment for calcium reabsorption in renal tubules. Its cells create a water-proof barrier, actively transport sodium and chlorine through a transcellular pathway, and provide a paracellular pathway for selective calcium reabsorption. Several studies have led to a model of paracellular channel consisting of various claudins, particularly claudin-16 and 19. Claudin-16 mediates cationic paracellular permeability in ATLH, whereas claudin-19 increases cationic selectivity of claudin-16 by blocking anionic permeability. Recent studies have shown that claudin-14 promoting activity is only located in ATLH. When co-expressed with claudin-16, claudin-14 inhibits the permeability of claudin-16 and reduces paracellular permeability to calcium. Calcium reabsorption process in ATLH is closely regulated by calcium sensor receptor (CaSR), which monitors circulating Ca levels and adjusts renal excretion rate accordingly. Two microRNA, miR-9 and miR-374, are directly regulated by CaSR. Thus, miR-9 and miR-374 suppress mRNA translation for claudin-14 and induce claudin-14 decline.
Los canales paracelulares que se encuentran en las uniones estrechas tienen un papel fundamental en los flujos iónicos transepiteliales. Esta vía está formada por un gran número de proteínas, entre ellas, las claudinas. En el epitelio renal, las claudinas confieren selectividad iónica a la unión estrecha. La rama gruesa ascendente de Henle (RGAH) es el segmento tubular renal más importante en la reabsorción tubular de calcio. Sus células forman una barrera impermeable al agua, transportan activamente sodio y cloro por la vía transcelular y proveen una vía paracelular para la reabsorción selectiva de calcio. Varios estudios han llevado a un modelo en el que distintas claudinas forman el canal paracelular, especialmente la claudina 16 y 19. La claudina 16 media la permeabilidad paracelular catiónica en la RGAH mientras que la claudina 19 incrementa la selectividad catiónica de la claudina 16 bloqueando la permeabilidad aniónica. Recientemente se ha encontrado que la actividad promotora de la claudina 14 está localizada exclusivamente en la RAGH. Cuando se coexpresa con la claudina 16, la claudina 14 inhibe la permeabilidad de la claudina 16, reduciendo la permeabilidad paracelular al calcio. El proceso de reabsorción de calcio en la RGAH está estrechamente regulado por el receptor sensor de calcio (CaSR) que monitorea los niveles circulantes de Ca ajustando la tasa de excreción renal de forma acorde. Dos micro-ARN, los mir-9 y mir-374, son regulados directamente por el CaSR. Los miR-9 y miR-374 suprimen la traslación del ARNm de la claudina 14 e inducen su decaimiento.
Epithelial transport can occur by the transcellular route, across epithelial cells, or via a paracellular route between epithelial cells. In the last decade, evidence has accumulated supporting the fundamental role of paracellular channels in transepithelial flux of ions. The paracellular channel or route is found in the tight junctions (zonulae occludentes) of the epithelium in vertebrates. Tight junctions are the most apical structure of the intercellular junctional complex. Tight junctions are composed of a large number of different proteins. Of those, membrane proteins probably play a central role in determining paracellular permeability, as their extracellular domains protrude into the paracellular space, with an ideal position to influence paracellular movement of solutes. The cell membrane proteins of the tight junction include occludins, junctional adhesion molecules (JAM), and claudins. Claudins include a large family of at least 26 proteins that were first identified in 1998.1 It was subsequently found that claudin-16, also known as paracellin-1, was mutated in familiar hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC).2
FHHNC appeared to be due to a defect in paracellular reabsorption of calcium and magnesium in the thick ascending limb of the loop of Henle (TAL). This was a first indication that claudin-16, and by extension claudins in general, might play an important role in the paracellular ion permeability of the kidney. In a recent genomic association study, the gene for claudin-14 was identified as being associated with increased risk for development of hypercalciuric nephrolithiasis,3 making this protein another candidate for involvement in reabsorption of bivalent cations. All this information led us to re-examine the significance of claudins present in the kidney and their regulation of tubular calcium reabsorption.
Claudin structureClaudins are 21 to 28kDa proteins with 4 transmembrane domains, 2 extracellular loops, 2 cytoplasmic domains–one amino and one carboxyl terminal–and a short cytoplasmic loop. The first extracellular loop (ECL1) of claudins consists of approximately 50 amino acids with a common motif (GLWCC). It contains positively and negatively charged amino acids. The charges on ECL1 regulate ion selectivity by means of electrostatic effects. The second extracellular loop (ECL2) consists of approximately 25 amino acids with a predicted helix-loop-helix motif that mediates the intracellular interactions of claudins. The C-terminal domain contains the PDZ binding domain that is critical for interaction with the submembrane protein ZO-1 and the correct localisation of the claudin in the tight junction. In the renal epithelium, claudins have been shown to confer ion selectivity to the tight junction, resulting in differences in transepithelial resistance and paracellular permeability. For example, claudin-4, 5, 8, 11, and 14 selectively decrease tight junction permeability to cations, particularly to sodium, potassium, hydrogen, and ammonium, whereas claudin-2, 15, and 16 increase permeability to cations, particularly sodium, potassium, calcium, and magnesium.4
Claudin expression in different tubular segmentsMost claudins are expressed in the renal tubule. Each segment and cell type expresses multiple isoforms. It is thought that the particular group of claudins expressed in each tubular segment determines the unique permeability properties of those segments.5 Claudin-2 is highly expressed in the proximal tubule, mainly in the terminal part of the proximal tubule and the initial part of the thin descending loop of Henle; the fundamental role is to form paracellular cation-selective pores with high sodium conductivity. Claudin-10a and claudin-17 are both known to form anion-selective paracellular pores and are potential candidates for mediating paracellular reabsorption of chloride in the distal part of the this tubular segment. Claudins-16 and 19 are expressed in the thin and thick ascending limb of the loop of Henle and are clearly required for the paracellular reabsorption of bivalent cations. Some investigators think that these 2 claudins form the paracellular pore that mediates permeability to calcium and magnesium in the TAL. Others, such as Hou et al., have found that claudin-16 increases permeability to sodium, whereas claudin-19 reduces chlorine permeability, generating a dilution potential that drives paracellular calcium and magnesium movement. In the aldosterone-sensitive segment of the distal nephron, where active reabsorption of sodium and secretion of potassium and hydrogen ions take place, the main role of the paracellular pathway is to act as a cation barrier to prevent backleak of the actively-transported cations. In this segment, claudin-3, 4, 7, 8, and 10 are expressed. Claudins 4 and 8 act as cation barriers and interact in such a way that claudin-8 is required for claudin-4 to be assembled within the tight junction. Claudin-7 likely behaves as a Cl− pore, being responsible for paracellular Cl− conductivity in this tubule segment.
Renal calcium handlingOn average, the kidneys reabsorb 97–99% of the daily filtered load of calcium. Of this reabsorbed calcium, 60–65% is reabsorbed in the proximal tubule via the paracellular route and 25–30% is absorbed in the TAL, also paracellularly. The remaining 8% to 10% of filtered calcium is absorbed in the distal convoluted tubule, via the transcellular route.6 This transcellular transport consists of 3 stages: first, the apical entry of calcium via the TRPV5 (transient receptor protein-vanilloid 5) channel; second, the intracellular diffusion of calcium from the apical membrane to the basolateral membrane, bound to a protein called calbindin-D28K; and finally, the exit of calcium across the basolateral membrane by means of a Na/Ca exchanger (NCX1) and a calcium ATPase (PMCA1b).7
Proximal tubular reabsorption of calciumThe proximal tubule of the adult kidney is a highly-permeable membrane that reabsorbs up to two thirds of the total filtered chloride load as well as two thirds of the ultrafiltrate volume. Almost half of all NaCl reabsorption is paracellular. The first portion of the proximal tubule reabsorbs bicarbonate-bound sodium preferentially over chloride-bound sodium, with associated reabsorption of water. This means the tubular fluid arriving at the final part of the S1 segment and the S2 and S3 segments of the proximal tubule has a higher chloride concentration than the peritubular liquid. In these sections of the proximal tubule, the paracellular route is preferentially permeable to chloride, which is reabsorbed by passive diffusion following the concentration gradient, in turn generating a lumen-positive potential, which drives paracellular sodium reabsorption. Claudin-2, which has been shown to act as a paracellular cationic pore,8 is highly-expressed in the proximal tubule9 and the thin descending limb of the loop of Henle, showing an axial increment in its levels of expression. Claudin-2 has been shown to be capable of transporting potassium and calcium, making it an excellent candidate for a paracellular pore that allows cation reabsorption. This was confirmed by Muto et al., using a knockout model for claudin-2 in mice.10 The mice had decreased cation permeability and a reduction in NaCl and water reabsorption when measured in isolated perfused proximal tubules. In balance studies in whole animals, the fractional excretions of sodium and chorine were comparable to those of wild mice in normal conditions. However, when they were given a high-salt diet, the excretions were significantly higher. These knockout mice did not have substantial changes in K metabolism but were hypercalciuric, suggesting that claudin-2 could mediate paracellular calcium reabsorption in the proximal tubule. In addition to claudin-2, the proximal tubule expresses claudins-10a, 12, and 17. Claudins-10a and 17 could function as anion-selective pores and be responsible for paracellular chlorine reabsorption. At an intestinal level, claudin-12 is regulated by vitamin D and functions as a calcium-selective pore. In the proximal tubule it could play a similar role, along with claudin-2.
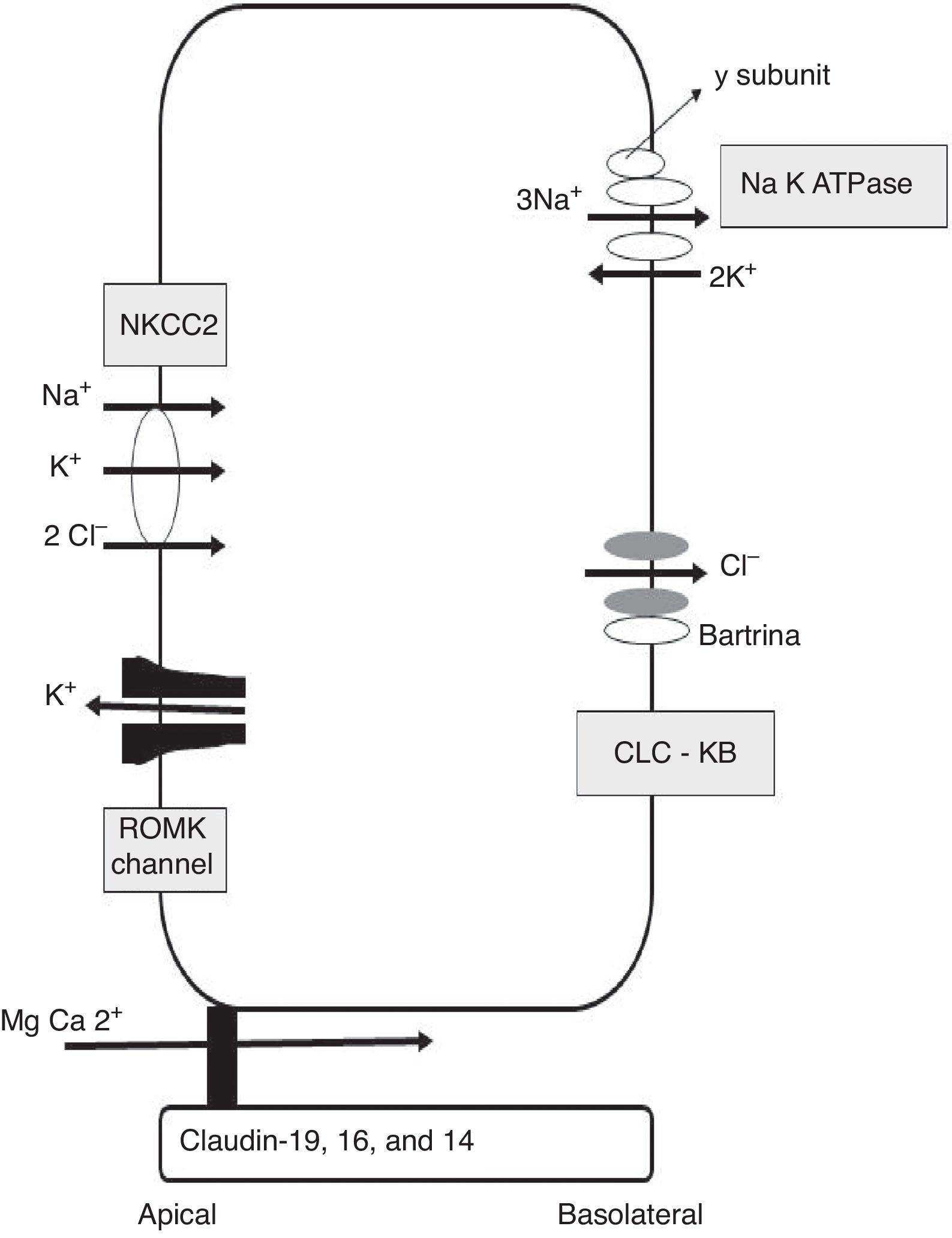
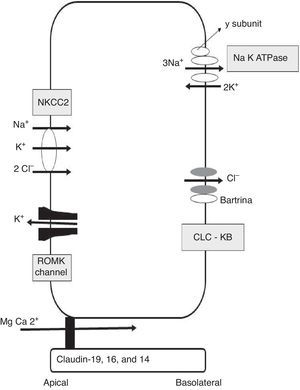
Calcium reabsorption in the thick ascending limb of the loop of HenleThe TAL is the most important renal tubular segment in terms of tubular calcium reabsorption. The epithelial cells that line the TAL form a barrier that is impermeable to water, with an active transcellular transport of sodium and chlorine, providing a paracellular route for selective reabsorption of calcium. Calcium is passively reabsorbed from the lumen to the interstitial space via the paracellular route, driven by a lumen-positive transepithelial voltage gradient11 (Fig. 1). Generation of this transepithelial voltage is attributed to 2 mechanisms: 1) apical potassium secretion through the renal outer medullary potassium (ROMK) channel, and basolateral secretion of chlorine through chlorine Kb (ClC-Kb) and bartrin channels, driven by apical NaCl reabsorption via the Na2ClK cotransporter (NKCC2); and 2) the transepithelial diffusion voltage generated by the transepithelial concentration gradient of NaCl around the cation-selective paracellular channel in the TAL. In the first segment of the TAL, the first mechanism provides a voltage of around +8mV, with minimal contribution from the diffusion potential in this early segment because the concentration gradient has not yet developed. With the continued reabsorption of NaCl along the axis of the TAL, the luminal fluid is diluted and a high concentration gradient is generated from the peritubular space to the tubular lumen of the distal segment of the TAL. As the paracellular permeability of the TAL is cation-selective, the positive transepithelial diffusion voltage is superimposed on the active transport transepithelial voltage, transforming into the main driving force for calcium reabsorption through the paracellular channel, with a voltage now substantially increased up to +30mV.
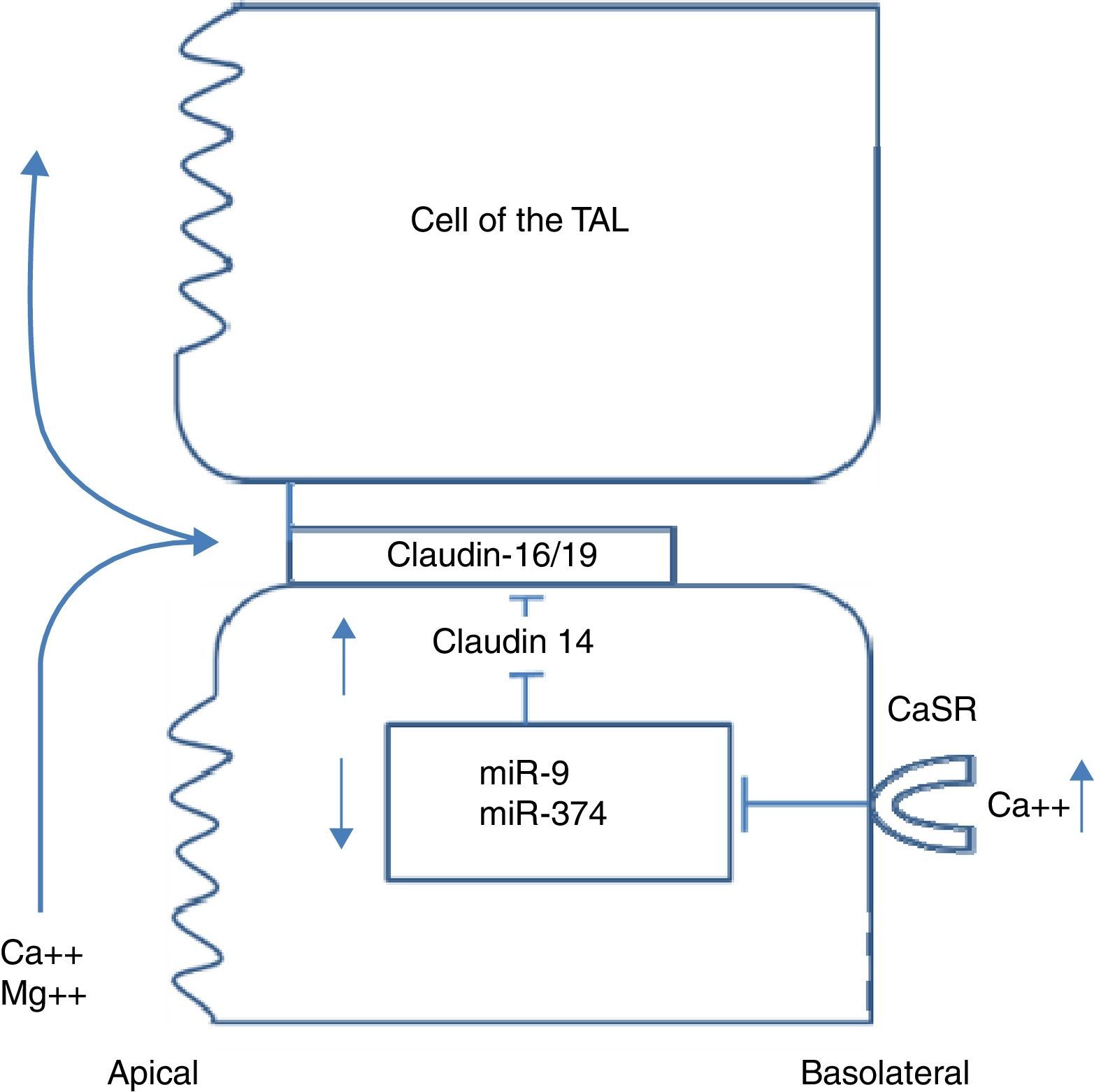
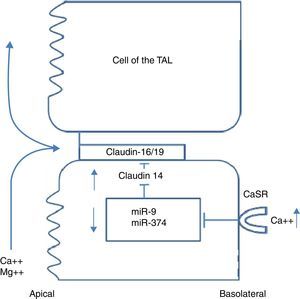
Claudins in the paracellular channel of the thick ascending limb of the loop of HenleSeveral studies have led to a model in which claudins form paracellular channels,12 in particular 2 of them: claudin-16, also known as paracellin-1, and claudin-19. Whilst claudin-16 is expressed only in the TAL of the kidney, claudin-19 has a broader expression, being found not only in the TAL of the kidney but also in the pigmented epithelium of the retina. Claudin-16 mediates paracellular cation permeability in the TAL.13 Claudin-19 increases the cation selectivity of claudin-16, blocking anion permeability.14 Paracellular cation selectivity is required to generate the lumen-positive transepithelial diffusion voltage that drives reabsorption of calcium and magnesium in the TAL.12 Patients with FHHNC have mutations in claudins-16 and 19. The phenotypic similarity of both mutations is explained by the direct interaction between the two proteins.15 However, claudin-19 mutations are invariably accompanied by severe ocular anomalies (including severe myopia, nystagmus, and macular coloboma), therefore this phenotype is known as FHHNC with severe ocular involvement.16 Recently, a genome-wide association analysis study was performed on 37734 patients with hypercalciuric lithiasis and 42510 control participants without lithiasis, in Iceland and The Netherlands.3 Four common synonymous variants at the locus of the claudin-14 gene (single nucleotide polymorphisms [SNP]) were significantly associated with renal lithiasis. Two of the variants were non-exonic and 2 were exonic. Both exonic SNPs were significantly associated with reduced bone mineral density. Urinary calcium excretion was higher in homozygous carriers of one of those polymorphisms than in non-carriers. Until recently, the localisation of claudin-14 at a renal level was disputed, until Gong et al. found that claudin-14 promoter activity was located exclusively in the TAL of mouse kidneys.17 In mice fed a normal diet, both claudin-14 mRNA and protein levels were extremely low. However, mice fed a high-calcium diet showed a marked increase in claudin-14 mRNA and protein levels in the TAL. In keeping with previous findings, claudin-14 works as a paracellular cation barrier. When co-expressed with claudin-16, claudin-14 inhibits the permeability of claudin-16, which is of great significance as a paracellular cation channel of the TAL. The knockout mice for claudin-14 had normal renal function under normal dietetic conditions. However, their kidneys excreted significantly less calcium and magnesium than wild mice when they were fed with a calcium-rich diet.17 The observed association between claudin-14 and nephrolithiasis could be explained by a dysregulation of claudin-14 that blocks the claudin-16 channel, producing a variable phenotype similar to that of FHHNC
Integrated signalling system that controls calcium transport in the thick ascending limb of the loop of HenleAs previously mentioned, the TAL is the main segment responsible for tubular reabsorption of calcium. The epithelial cells that make up the TAL form a barrier that is impermeable to water, actively transport NaCl via the transcellular route, and provide a paracellular channel for reabsorption of cations, including calcium. Paracellular calcium reabsorption is driven by a lumen-positive transepithelial voltage. There are 2 prerequisites for generation of this gradient: 1) a significant transepithelial NaCl gradient, dependent in the coordinated action of the Na/K/2Cl cotransporter (NKCC2) and the ROMK potassium channel, both in the apical membrane, and the chlorine channel (ClCKb-bartrin) in the basolateral membrane (Fig. 1); and 2) a cation-selective paracellular channel dependent on the interaction of claudin-16, 19, and 14. Monogenic diseases such as Bartter syndrome and FHHNC are caused by mutations in the genes underlying both of these prerequisites. The process of calcium reabsorption in the TAL is tightly regulated by the calcium-sensing receptor (CaSR), which monitors circulating calcium levels, adjusting the rate of renal excretion accordingly.18 Recently, it was demonstrated that CaSR regulates calcium reabsorption by changing paracellular, not transcellular, permeability to calcium.19 When CaSR is activated by a high dietary intake of calcium or by induction of hypercalcaemia due to prolonged calcitriol administration, claudin-14 expression in the TAL increases.19 In keeping with this, CaSR activation by administration of the calcimimetic cinacalcet leads to a 40-fold increase in claudin-14 mRNA.20 Furthermore, in 2 separate cell culture models, the overexpression of claudin-14 reduced paracellular calcium flux, thus reducing cationic permeability. Using animals that express Cre recombinase driven by the Six2 promoter, mice with undetectable levels of CaSR mRNA in the kidney were created.21 These mice had urinary calcium levels lower than controls when they were challenged with a calcium-supplemented diet. This was associated with a significant reduction in claudin-14. Therefore, the activation of CaSR in the TAL increases claudin-14 expression, which in turn blocks paracellular calcium reabsorption.20 Two micro-RNA that are directly regulated by CaSR in the cells of the TAL have also been identified: miR-9 and miR-374.16 miR-9 and miR-374 recognise partially-complementary binding sites located at the 3′-UTR of claudin-14 RNA, suppressing their translation and inducing synergistic mRNA decay. Under normal dietary conditions, miR-9 and miR-374 repress the level of genetic expression of claudin-14 and protect the claudin-16 function in the paracellular channel. With a high calcium ingestion, CaSR is activated and down-regulates expression of miR9 and miR-374, causing a reciprocal increase in claudin-14 expression. The increase in claudin-14 proteins in the tight junctions inhibits the cation selectivity of claudin-16 in the paracellular channel, reducing calcium reabsorption in the TAL. Gong et al. recently reported that administration of histone deacetylase (HDAC) inhibitors reduced claudin-14 messenger RNA and drastically reduced urinary calcium excretion in mice.22 Furthermore, treatment with HDAC inhibitors stimulates transcription of the genes coding for micro-RNA-9 and micro-RNA-374. These mRNAs that have been demonstrated to repress expression of claudin-14, the negative regulator of the paracellular pathway of calcium reabsorption (Fig. 2).
ConclusionClaudins have been recognised as critical molecules in the regulation of paracellular calcium reabsorption at a renal level. In particular, claudin-14, 16, and 19 form paracellular channels in the TAL that regulate reabsorption of calcium and magnesium in this portion in the renal tubule. They are in turn regulated by the calcium-sensing receptor. The mutations and polymorphisms that affect the genes coding for these proteins appear to produce a dysregulation of urinary calcium excretion. This knowledge on the regulation of the paracellular pathway by CaSR using micro-RNA and its modification by HDCA inhibitors allows us to envisage new future treatments for hypercalciuric diseases.
Conflicts of interestThe author declares no conflicts of interest.
Please cite this article as: Luis Negri A. Rol de las claudinas en el manejo renal del calcio. Nefrologia. 2015;35:347–352.