To the Editor,
The prognosis of Wegener’s granulomatosis (WG) has improved with current therapies, but there is still a group of patients refractory to treatment. We describe a case of a refractory WG patient that reached remission with plasmapheresis and rituximab therapy, but later developed colon adenocarcinoma.
Immunosuppressive agents are effective drugs, but they are not exempt from severe secondary effects, such as infections and neoplasias. Our patient received different immunosuppressive treatments, among which was rituximab, which is considered to be an effective drug, but its long-term safety profile is unknown. Clinical monitoring is important in patients that receive multiple immunosuppressant drugs, given that they have a high risk of developing malignant diseases.
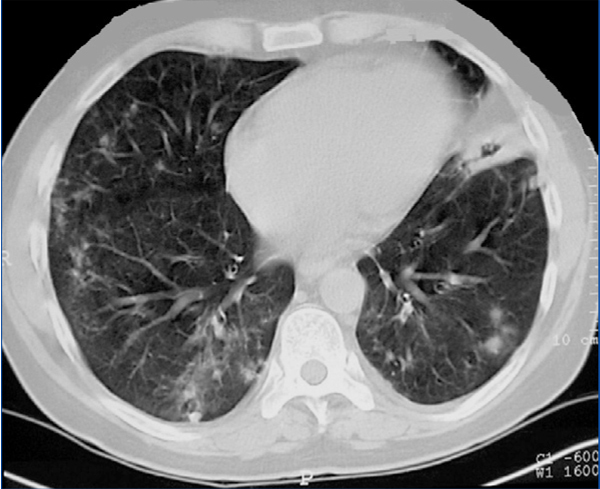
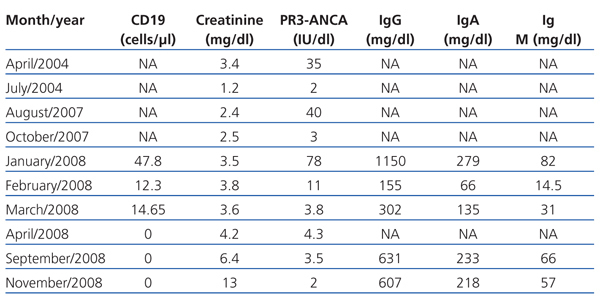
We present the case of a 42-year-old man, diagnosed with WG in 2004 by a renal biopsy. Induction treatment with 6-methylprednisolone boli (50mg) IV for three consecutive days, followed by oral prednisone (1mg/kg/day) and oral cyclophosphamide at doses of 100mg/day (1.5mg/kg/day). After three months of treatment, the patient achieved clinical remission, and PR3-ANCA became negative. During the maintenance phase, cyclophosphamide was replaced with mycophenolate mofetil (720mg/12hr). In 2007, the patient suffered a relapse. Treatment with cyclophosphamide in intravenous boli at doses of 850mg (10mg/kg) was resumed, with oral prednisone (1mg/kg/day). Clinical manifestations remitted and PR3-ANCA became negative after two months of treatment. However, in January 2008, the patient was admitted with a second relapse with haemoptysis and renal failure. He was administered 1g of intravenous cyclophosphamide every three weeks together with oral prednisone (1mg/kg/day). The patient continued with clinical respiratory and renal problems despite having received an accumulated dose of cyclophosphamide of 16g and high doses of corticosteroids. He was therefore diagnosed with refractory Wegener’s granulomatosis. A second kidney biopsy was performed, which showed persistence of pauci-immune necrotising glomerulonephritis with an extracapillary reaction in 7% of the glomeruli, moderate interstitial fibrosis and tubular atrophy and glomerulosclerosis in 50% of all glomeruli. Given the haemoptysis’ respiratory clinical involvement (Figure 1), plasma refills were started every other day for 15 days and oral prednisone at a dose of 1mg/kg/day. After the plasma refills were finished, rituximab was administered (375mg/m2 a week) for four weeks. Treatment with rituximab was monitored, measuring B lymphocytes in peripheral blood through CD19, immunoglobulin levels and PR3-ANCA titre (Table 1). Respiratory symptoms improved and renal function stabilised (creatinine 3.6mg/dl). All inflammation markers decreased, PR3-ANCA became negative and no B lymphocytes were found in peripheral blood. Mycophenolate mofetil was started with low doses of prednisone as a maintenance treatment. In November 2008, he was admitted again for anaemia-inducing rectal bleeding and progressive chronic renal failure with no signs of vasculitis activity (negative PR3-ANCA). He was diagnosed with rectal adenocarcinoma and had to start on haemodialysis.
Ten months later, the patient remained on haemodialysis, with no signs of vasculitis, negative PR3-ANCA and CD19 in peripheral blood at 0%.
Treatment with cyclophosphamide and corticosteroids has improved the prognosis of vasculitis, achieving remission for between 70% and 90% of cases.1 Nonetheless, 10% of patients do not achieve remission or present with frequent relapses, and are considered to be refractory to treatment.2 Several alternative treatments have been described for cases of refractory WG, such as: infliximab, etanercept, 15-deoxyspergualin, leflunomide, anti-lymphocyte serum and rituximab. Unfortunately there are not enough studies that indicate the best alternative therapy.3
Rituximab is a chimeric monoclonal antibody against the CD20 antigen that is expressed on the B lymphocyte surface; its binding results in the elimination of B lymphocytes and inhibition of antibody synthesis.4 At present there are several studies on its effectiveness in some autoimmune diseases and its use has also been described in a series of patients with ANCA-positive vasculitis refractory to treatment, in which full remission rates of 75% have been observed.5 In most studies with cases of refractory vasculitis which used rituximab, it was used concomitantly with steroids or other immunosuppressive agents. Patients achieved clinical remission in an average time of 2-6 months and in most cases remission was achieved together with a B-lymphocyte depletion in peripheral blood and a significant reduction in ANCA titres. B-lymphocyte recovery in peripheral blood often occurs at an average of 9-11 months and this is a risk factor for relapses after the first rituximab cycle. Repeated rituximab doses have been described, both as a preventative measure when B lymphocytes are recovered, and in relapse cases. This also achieves high levels of remission.6 Many of the studies on rituximab treatment consider it to be a safe and effective drug. The most common secondary effects are related to drug infusion. Among other less common effects are: development of neutropaenia, infections, progressive multifocal leukoencephalopathy, reactivation of hepatitis, intestinal perforation and interstitial pneumonitis, but there are few data that relate it to neoplasia development.7 It is difficult to be able to obtain conclusions on the safety of rituximab to treat vasculitis, given that the studies published are of reduced size. Recently, a study was published comparing rituximab with cyclophosphamide in the induction phase for patients with ANCA-positive vasculitis. It observed 6 cases of solid organ neoplasia in the group treated with rituximab (2 cases of colon neoplasia), which represented 5% compared to the control group.8
Our patient did not have any family or personal history of malignant diseases, but developed a colon adenocarcinoma. Patients who have accumulated high doses of cyclophosphamide are at risk of developing neoplasias, according to the literature.9 Our patient received high doses of cyclophosphamide, but we were not able to confirm that the colon neoplasia was only related with this treatment. We suggest that rituximab could have been involved in the development of the colon adenocarcinoma. We believe that clinical monitoring is important in patients that receive multiple immunosuppressive drugs, given that they have a high risk of developing malignant diseases.
Figure 1. Chest computerised tomography showing pulmonary bleeding
Table 1. Evolution of biochemical parameters over time