Introducción: generalmente, se ha aceptado que el riñón no sea un órgano afectado en la Fibrosis Quística (FQ). Diferentes estudios han demostrado que los pacientes con FQ tienen una mayor predisposición a padecer nefrolitiasis por cálculos de oxalato cálcico que la población general. Objetivo: estudiar la prevalencia autonómica de enfermedad renal en pacientes con FQ. Pacientes y métodos: estudio transversal analítico de ámbito autonómico, con grupo control. Grupo índice: 20 pacientes con FQ de 4-30 años de edad. Grupo control: 73 sujetos sanos, seleccionados al azar entre los 100 individuos de 13-21 años de edad que completaron el seguimiento longitudinal del estudio RICARDIN. Realización en ambos grupos de estudio de examen físico y pruebas analíticas de función renal, repitiendo en el grupo FQ la recogida de datos 20 meses después. Ecografía renal sólo en el grupo índice. Resultados: el filtrado glomerular estimado por la talla no alcanzó diferencias significativas en el grupo FQ respecto al control. Los pacientes del grupo FQ presentaron valores significativamente más altos de oxaluria (0,77 mg/kg/24 h, p = 0,001) y fosfaturia (18,98 mg/kg/24 h, p = 0,04), y valores más bajos de citraturia (7,76 mg/kg/24 h, p = 0,04) y magnesuria (1,60 mg/kg/24 h, p = 0,04) que los valores normales de referencia. La ecografía renal no demostró en ningún caso hallazgos sugerentes de nefrocalcinosis ni nefrolitiasis. Conclusiones: los pacientes con FQ presentan una función renal conservada, sin diferencias significativas con los controles. Estos pacientes presentan alteraciones en la excreción urinaria de solutos que pueden favorecer la formación futura de cálculos renales.
Introduction: It has been generally admitted that kidneys are not affected in Cystic fibrosis (CF) patients. Anyway, there are several studies demonstrating a higher prevalence of calcium oxalate nephrolithiasis prevalence in CF patients compared to normal population. The aim of our study was to evaluate renal disease regional prevalence in CF patients Methods: Cross-sectional regional controlled study. Index group = 20 CF subjects 4- 30 years old controlled in a CF outpatient clinic in a tertiary hospital. Control group = 73 healthy subjects randomly selected among the 100 subjects who completed the follow-up visits of the RICARDIN study. Physical examination and renal function analysis were conducted in both groups. CF patients had their functional measurements repeated in a 20 month interval. Renal ultrasonography was performed only in CF patients. Results: Height estimated creatinine clearance in CF patients were not statistically different from controls. Oxaluria (0.77mg/kg/24 h, p = 0.001) and phosphate excretion (18.98 mg/kg/24 h, p = 0.04) were significantly higher while citrate (7.76mg/kg/24 h, p = 0.04) and magnesium (1.60mg/kg/24 h, p = 0.04) excretion were significantly lower in CF patients than normal population reference values. No signs of nephrolithiasis or nephrocalcinosis were found. Conclusions: CF patients showed a conserved renal function , without ecographic abnormalities. CF patients showed increased urinary elimination of phosphate and oxalate and lower citrate and magnesium elimination, findings that predispose these patients to suffer from urolithiasis in the future.
INTRODUCTION
Pancreatic Cystic Fibrosis (CF), or mucoviscidosis, is the most common fatal hereditary illness amongst the Caucasian population. It affects approximately 1 out of 2,500 new-born infants.1 It is an autosomal recessive, multisystemic disorder caused by a mutation of the gene which codes for a protein referred to as the CF transmembrane conductance regulator (CFTR). This protein is located in the long arm of chromosome 7, at position 7q31.2
There are more than 1,000 different mutations that can cause CF, although around 80% of mutations result from the mutation called ΔF508, consisting of the deletion of three base pairs in CFTR's nucleotide sequence. This deletion causes loss of the amino acid phenylalanine located at position 508 in the protein. Among other symptoms, this mutation causes excessive sodium chloride to be lost through perspiration, which is a key symptom in diagnosis of the disorder.3 The resulting sodium and chloride ion imbalance causes abnormally thick mucus secretions, which lead to respiratory and digestive infections, key factors in the prognosis of CF patients.
Clinical manifestations of CF encompass nearly all of the organs and systems in the body. Among others these may include: the digestive tract (meconium illeus [MI], intestinal obstruction), the bile ducts (cholelithiasis, biliary cirrhosis), the pancreas (exocrine pancreatic insufficiency [EPI], diabetes mellitus) and the genitourinary system (azoospermia.)
Traditionally, it has been accepted that the kidneys were not affected by CF and up until now there has been no demonstrable change in renal function among CF patients. However, the CFTR protein expresses in the renal epithelium4 and is detected in the proximal tubule, Henle’s loop, distal tubule and collecting ducts.
Various studies have shown that there is a higher prevalence of calcium oxalate nephrolithiasis among CF patients than amongst the rest of population. Specialist literature describes a prevalence of 3.5-5.7% in CF patients compared to 1% in the general population.5-7Although urolithiasis is usually observed in CF patients over the age of 20, it is not unusual to observe it in children.
Furthermore, signs of nephrocalcinosis have frequently been found in anatomopathological studies on the kidneys of CF patients.8 Nephrocalcinosis was discovered in 35 out of 38 samples of the renal material of CF patients, suggesting that CF causes an abnormality in the metabolism of calcium. Other studies, however, dispute these results.9 The mechanisms that promote this greater prevalence of nephrolithiasis and nephrocalcinosis have not been sufficiently explained yet.
The objective of this study was to analyse the prevalence of renal illness in a group of CF patients controlled in a regional unit.
PATIENTS AND METHODS
Study group: 20 CF patients (nine males) aged between 4 and 30, controlled in the CF outpatient clinic of a tertiary hospital (Asturias Central University Hospital.) The patients were examined on two separate occasions at an interval of 20 months. In order to be included in the study, personal or parental authorisation was obtained following a detailed explanation of protocols to the patients and their families. Exclusion criteria: any CF patient suffering from acute or aggravated illness over and above their normal condition.
Control group: 73 healthy adolescents (43 males) aged between 13 and 21 selected at random from among a sample of 100 individuals who completed the RICARDIN10 study follow-up visits. This was a multi-centre study carried out on 14,000 children in fourteen different locations in Spain to investigate the prevalence of different cardiovascular risk factors in childhood and to produce Blood Pressure (BP) reference tables.
Weight (kg), height (m), Body Mass Index (BMI) (weight [kg]/height2 [m2]), arm circumference (cm) and tricipital fold (mm) were all determined. Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP) were measured in both groups using the Erkameter® 3000 Mercurial Sphygmomanometer. RICARDIN10 study protocols, which demand that researchers be certified in BP determination, were adhered to. Patients rested for a period of five minutes prior to their BP being determined. Two BP measurements were taken at a minimum interval of five minutes with the subject seated and their arm resting on a table. The mean of the two measurements was used to assign a final BP value to each subject.
Blood analysis (automated methods): creatinine, ions, uric acid and osmolarity. Isolated urine sample analysis (automated methods): creatinine, sodium, chlorine, potassium and osmolarity. Following this, urinary concentration capacity and Fractional Excretions of Sodium (EFNa), Potassium (EFK) and Chrorine (EFCl) were calculated.
Analysis of 24-hour urine samples in CF group (automated methods): sodium, potassium, chlorine, calcium, phosphorus, magnesium, uric acid, oxalic acid and citric acid.
CF group patients underwent a new determination of all the analyses (somatometry, BP, blood and urine) after a period of 20 months. For statistical analysis purposes, the mean of the values obtained in the two separate determinations was calculated.
Creatinine clearance was calculated only for CF patients, using the conventional formula corrected for adult body surface area (1.73 m2): Ccr (ml/min 1,73m2) = Cr urine/Cr plasma x volume per minute x 1.73/m2. Estimated Renal Function (ERF) was calculated for both study groups using the Schwartz et al.11 formula: Ccr (ml/min/1.73m2) = K x height (cm)/Cr in blood (mg/dl). The K-constant used was 0.50 for all children and 0.70 for adolescent males.
Normal values for renal function parameters in adults and children are considered to be those in recent publications by the Spanish Paediatric Nephrology Association and in the Nephrological diagnostic and therapeutic protocols published by the Spanish Paediatric Association (Asociación Española de Nefrología Pediátrica)12-14 as well as figures published by Böhles H et al.15 in a recent study carried out on 96 CF patients and 30 controls. All CF patients in the study group underwent abdominal ultrasonography to assess renal size from the length of the three renal diameters and to calculate renal volume by multiplying the three values measured in both kidneys. Subjective images evidencing renal lithiasis, nephrocalcinosis or any other renal disorder were also investigated.
Statistical analysis: data was processed using SPSS 11.0 for PC. Basic statistical descriptive analysis techniques were applied to give a preliminary analysis of the results. To establish differences between the groups, the different variables analysed with appropriate statistics were compared (quantitative variables: Student’s t-tests and non-parametric tests where variable normality assumptions were violated; categorical variables: chi square tests.)
Multivariate adjustment techniques were used to control the confusion that the possible differences between groups might produce in different covariables. Values of p < 0.05 were considered to be statistically significant.
Funding: the study was carried out with the support of the following: Ernesto Sánchez Villares Foundation (Fundación Ernesto Sánchez Villares), 2002 call, Carlos III Health Institute (Instituto de Salud Carlos III), (Reference FIS 03/0535.)
RESULTS
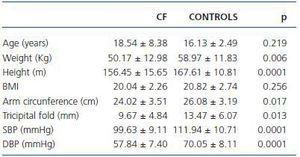
The CF patients presented somatometric values significantly lower than the control subjects (table 1), both for weight and height as well as for adiposity determinations. Despite the observation of lower BMI values, the differences were not statistically significant.
The CF patients presented SBP and DBP values significantly lower than those of the control subjects (table 1.) A multivariate adjustment was performed which included age, sex, height and weight. After this adjustment, the differences in SBP and DBP did not prove to be statistically significant.
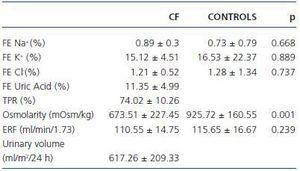
Different parameters of renal function were compared in both study groups (table 2.) The CF group patients presented values comparable to those of the control subjects for fractional excretion of sodium, chloride and potassium. Furthermore, the CF patients presented height estimated glomerular filtration rates that were similar to those of the control group (table 2.) Significantly lower urinary osmolarity was observed in the CF patients.
In addition, a reduced urinary volume was observed with values below 700ml/m2/day being demonstrated among almost three in five of the CF patients.
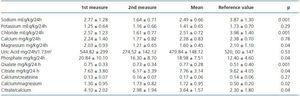
The differences in urinary solute excretion among CF patients were analysed alongside published reference values.
Oxaluria (0.77mg/kg/24h, p = 0.001) and phosphaturia (18.98mg/kg/24h, p = 0.04) were significantly higher, while citraturia (7.76mg/kg/24h, p = 0.04) and magnesuria (1.60mg/kg/24 h, p=0.04) were significantly lower in CF patients than normal population reference values. Urinary excretion values for calcium, urate and potassium were similar, whilst 24 hour sodium excretions were significantly lower in CF patients than the reference values.
Different quotients were calculated, the CF patients presenting significantly higher ratios of calcium/magnesium and citrate/calcium than the reference values. No significant differences in calcium/creatinine ratios were observed.
The renal ultrasonography showed no signs of nephrocalcinosis or nephrolithiasis.
DISCUSSION
Necropsy studies have detected the presence of nephrocalcinosis in up to 92% of CF patients, including those under the age of one.8 Furthermore, epidemiological studies have shown a nephrolithiasis prevalence of approximately 6%.7 Although urolithiasis is usually observed in CF patients over the age of 20, it is not unusual to observe it in children.
The mechanism responsible for lithogenesis in these patients has not been completely determined. In the current study a series of alterations in the urine of CF patients was demonstrated. These caused conditions which favour the development of kidney stones. CF patients evidenced greater oxaluria and phosphaturia than the general population. In addition, they presented less citraturia, magnesuria and daily diuresis.
The great majority of stones produced by these patients are composed of calcium oxalate.15 In this study oxaluria values in CF patients were significantly higher than the reference values. These findings coincide with the majority of studies carried out up until the present time.15-18 People with ileal resections, jejunoileal bypasses, inflammatory bowel disease and CF often present enteric hiperoxaluria which is caused by malabsorption and pancreatic insufficiency. Malabsorption of fats in the intestine favours the union of bile salts with calcium forming non-absorbable compounds. Enteric oxalate, if not combined with calcium, is found in higher levels to be absorbed in the colon; this facilitates malabsorption of bile salts.19
70% of the oxalate created by the diet is broken down by specific bacteria (principally by Oxalobacter formigenes) in the intestine. The antibiotics that CF patients are frequently required to take, in order to control the respiratory infections they commonly suffer from, lead to a decrease in this intestinal flora.20 This prevents the breakdown of oxalate and increases its absorption,15,21 thereby causing hyperoxaluria, with a consequent increase in the risk of lithiasis.
As these patients get older, their oxalate levels in urine increase,20 given that treatment with pancreatic enzymes does not totally correct hyperoxaluria.
Unlike oxalate, magnesium and citrate are considered to be protective factors against lithiasis. In this study citraturia values significantly lower than the theoretical norm were found. This is a common occurrence.15-17,20,22 The citrate forms compounds with calcium and reduces the concentration of urinary ionic calcium. Moreover, it inhibits the crystallisation of calcium oxalate, converting it into a protective factor against the formation of stones in the urine.17
The hyperproteic content in the diet of these patients, as well as the intestinal loss of bicarbonate, bring about a reduction in urinary citrate concentration.22 The urinary citrate value in this sample is clearly abnormal when compared with the normal reference values in specialised literature.
Hypercalciuria and hypomagnesuria are also cited as factors which favour nephrolithiasis in CF patients,16 previous studies have given contradictory results.17,18,23-25 CF patients usually present normal or low levels of urinary calcium, which protects them against the formation of stones.17, 18 However, this is not sufficient, as the hyperoxaluria they present is a more powerful factor favouring crystallisation than hypercalciuria.18,20
Patients in this sample presented levels of magnesium in the urine which were lower than normal. Calciuria was also low compared to normal figures, although not significantly so, and the calcium/creatinine ratio did not demonstrate any statistically significant differences when compared to reference values.
Specialised literature describes CF patients as usually presenting an increase in uric acid in the urine, which may be a factor favouring lithiasis in these same patients.16,17,26 Hyperuricosuria may be related to the significant ingestion of pancreatic enzymes, increased catabolism and increased oxidative stress27,28 in these patients, although the significance of uric acid as a cause favouring lithiasis has decreased in recent years.27,29,30 This is confirmed by the fact that urinary urate excretion in CF patients in this sample was not significantly different to that among the control group.
Phosphaturia in the CF group was significantly higher than the normal reference value. This must also be considered as a factor favouring the formation of kidney stones,15 although in other studies24 these differences have not been confirmed.
A final factor related to the formation of kidney stones is the presence of poor diuresis which can favour the precipitation of different solutes in the urine. The decrease in urinary volume favours lithiasis in CF patients.31 This sample of CF patients did not exhibit a reduction in the ingestion of liquids in the days prior to the study and the climate, being that of northern Spain, was not hot. However, urinary volumes below 700ml/m2/day were recorded amongst almost three out of five CF patients.
CF patients present higher levels of water loss by evaporation through the skin and lungs and, on the other hand, the pancreatic insufficiency that they suffer causes a reduction in water absorption by the intestine. Both situations may be responsible for the decrease in urine production observed in these patients.31
None of the CF patients who underwent a renal ultrasonography showed signs of nephrocalcinosis or the presence of kidney stones. In two previous studies, with sample sizes of 3425 and 63 patients,16 only four cases of nephrocalcinosis, among the larger sample, were detected. Although the literature describes a nephrolithiasis prevalence of 3.5-5.7% in CF patients, in this study, using a sample of 20 patients, this was not confirmed. It is worth highlighting two factors as a possible explanation for these differences: on the one hand, the lower ages of the patients involved (between 4 and 30 years old) and, on the other hand, the reduced sample size. Extrapolating the reference prevalence of 5.5%, it would be reasonable to expect to see a maximum of one case of nephrolithiasis in this series.
In this study, estimated renal function and creatinine serum values had a relatively similar distribution in both groups. This confirms the presence of a conserved renal function in CF patients, a fact which is widely accepted.32-34
Serum and urinary sodium values were significantly lower in the CF group, whilst fractional excretion of sodium was similar in both groups. These findings are probably due to the excessive loss of sodium suffered by these patients.24,35-37 Occasionally, they may present a risk of hyponatraemia, which may require to supplement their diet with sodium chloride.38,39
In short, CF patients presented a conserved renal function, within normal limits. However, changes in the excretion of different solutes that favour the development of urolithiasis were detected in the urine of these patients. The increase in life expectancy of these patients will mean that in future the doctors monitoring them may have to deal with the diagnosis of these complications more often.
In light of the above, it is suggested that in CF patients who present episodes of abdominal pain nephrolithiasis be considered as a possible cause. Knowing the way in which kidney stones are produced in CF, it is important to carry out periodical studies on these patients in order to control the possible lithogenic factors previously mentioned, to correct possible intestinal malabsorption and to take dietary measures, such as reducing the ingestion of oxalate, introducing citrate supplements in certain cases and recommending an increased intake of liquids which may prevent the formation of kidney stones.
Table 1. Characteristics of the study groups
Table 2. Results of urinary analysis
Table 3. Results of urinary excretions of solutes in Cystic Fibrosis patients