To the Editor,
Calcineurin inhibitor-based (CNI) immunosuppression regimens have improved the outcomes of renal transplantation. Unfortunately, the use of CNI has been associated with interstitial fibrosis and tubular atrophy, affecting graft function and graft survival1. In order to avoid exposure to CNI, agents such as sirolimus (SRL) have emerged as new therapeutic options. Therapeutic strategies with SRL include the minimisation, suspension, elimination and total absence of CNI2.
Experiences with CNI-free SRL/mycophenolate mofetil (MMF)/ST immunosuppression have not obtained sufficient acute rejection (AR) prophylaxis3. The introduction of induction therapy improved AR rates and short-term efficacy (1-3 years) with contradictory results4-7. We previously reported excellent and satisfactory results after 1 and 3 years without CNI8,9 and we now present an observational and retrospective study of efficacy and safety after 5 years of the SRL/MMF/ST regimen compared with cyclosporine (CS)/MMT/ST and selective induction with basiliximab in 41 patients enrolled between May 2004 and January 2005.
The study design has previously been reported in detail8. In this report, the results were analysed in two populations: the intention-to-treat (ITT) population, which included all patients with a functioning graft, and the population on treatment (OT), which included patients who were maintained on the same original study immunosuppression regimen.
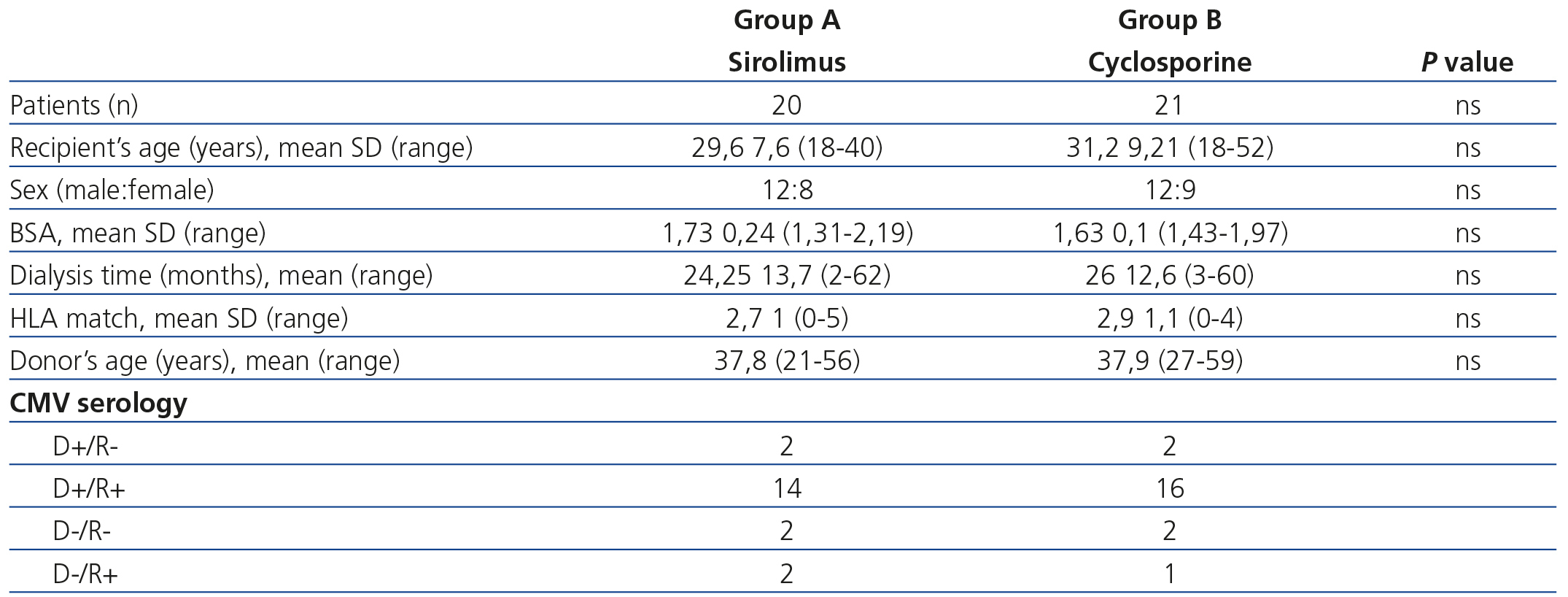
The demographic data of patients are displayed in Table 1. Five-year patient survival was 90% in the SRL group and 80.9% in the CS group (p=ns). The causes of death in the SRL group were cardiovascular (n=1) and infectious (n=1), which was similar to the CS group: cardiovascular (n=2), infectious (n=2) and gastrointestinal bleeding (n=1). Five-year graft survival was 80% for SRL and 76.1% for CS (p=ns). The causes of graft loss in the SRL group were: graft thrombosis (n=1), de novo glomerulonephritis (n=1), urological complications (n=1) and a lack of adherence to treatment (n=1). In the CS group they were: graft thrombosis (n=1), de novo glomerulonephritis (n=1), lupus (n=1), chronic kidney disease (n=1) and death with a functioning graft (n=1).
Eight patients (40%) from the SRL group and 3 (14%) from the CS group received basiliximab induction. After 5 years, there was a decrease in the dose of CS (133±29.9mg/day, range 120-200) and of SRL (1.75±0.66mg/day, range 1-3) compared to 12 months after transplantation (205.7±66mg/day and 3.2±1.7mg/day CS and SRL, respectively). The mean dose of MMF was higher in the CS group (1218.75±363g/day, range 500-2000), compared with the SRL group (1093.9±417g/day, range 500-2000) (p=.3). All patients in the study continued to take 5mg/day of oral prednisone. Four patients (25%) in the CS group (p=.039) with a functioning graft changed their regimen to SRL due to interstitial fibrosis and tubular atrophy confirmed by biopsy. We maintained all patients in the SRL group with a functioning graft on the SRL/MMF/ST regimen. After one year of follow-up, 2 patients in the SRL group (11.1%) and 3 in the CS group (17.7%) had episodes of AR (p=ns).
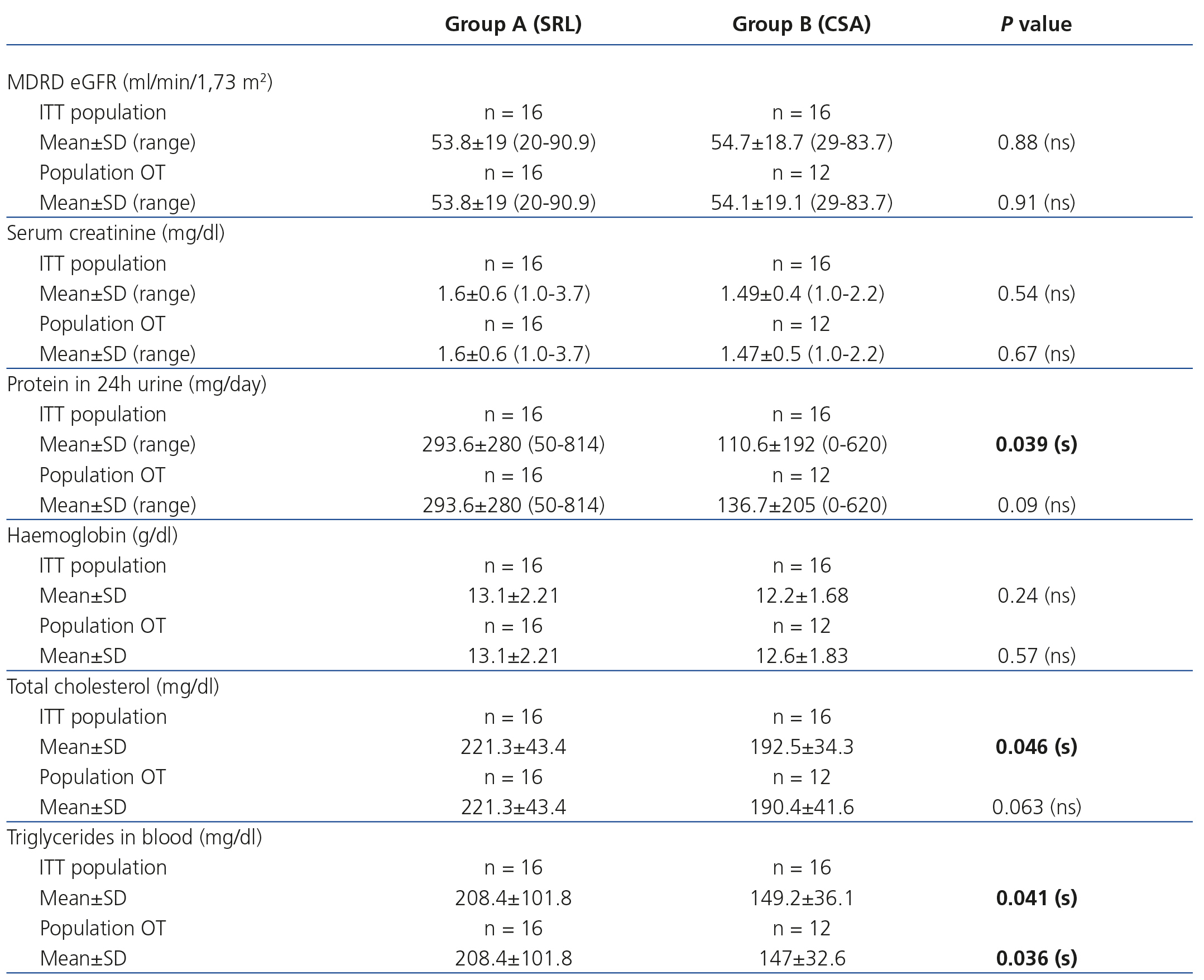
Graft function calculated by the glomerular filtration rate estimated using the MDRD (Modification of Diet in Renal Disease) formula10 and serum creatinine is displayed in Table 2. We did not find a statistically significant difference between the two groups, independently of whether they were an ITT population or a population OT. Patients in the SRL group had a higher elimination of proteins in 24h urine (p=.039) than patients in the CS group in the ITT population. Serum haemoglobin was similar in both cases. Cholesterol and triglycerides were significantly higher in the SRL group (Table 2).
There were a total of 81 adverse effect events, which were mostly infectious (14 in the SRL group and 16 in the CS group). There was a similar incidence in new onset diabetes after transplantation (NODAT) (10% in the SRL group versus 9.5% in the CS group). No patient developed a malignancy during follow-up. Six patients (37.5%) in the SRL group and 31.3% (n=5) in the CS group were taking angiotensin-converting-enzyme inhibitors and/or angiotensin receptor blockers after 5 years (p=.7). Similarly, more patients in the SRL group were taking lipid-lowering drugs than in the CS group (n=7, 43.8%, versus n=6, 37.5%) (p=.2).
In summary, despite the fact that our results need to be carefully reviewed due to certain limitations, such as the sample size, retrospective recording and a population of low immunological risk, we concluded that living donor transplantation patients with selective induction on the SRL/MMF/ST regimen have similar graft survival and function 5 years after transplantation to those on the CSA/MMF/ST regimen.
Conflict of interest
The authors declare the following conflicts of interest:
- Dr. Gustavo Martínez Mier receives lecture fees from Pfizer, Roche and Novartis and consultancy fees from Novartis and Sanofi.
Table 1. Clinical and demographic parameters
Table 2. Graft function based on the analysis of patients on treatment and those who we intended to treat