Los pacientes con enfermedad renal crónica terminal muestran precozmente resistencia insulínica (RI), caracterizada por alteraciones en el metabolismo hidrocarbonado e hiperinsulinemia generalmente asociada a dislipemia y a un patrón pro inflamatorio. La enfermedad cardiovascular (CV) constituye la principal causa de mortalidad en los pacientes en diálisis. Existe una fuerte asociación entre RI, hiperinsulinismo y enfermedad CV. El objetivo del presente estudio fue evaluar el efecto de la diálisis peritoneal (DP) sobre la RI y sus efectos sobre la morbimortalidad CV subsiguiente en pacientes urémicos no diabéticos. Se incluyeron 69 pacientes no diabéticos en DP, 35 incidentes (≤ 3 meses en DP) y 34 prevalentes (> 3 meses en DP), con 2 mediciones de resistencia insulínica estimada mediante el índice de resistencia a la insulina (HOMAIR), separadas entre sí por 12 meses. El valor medio de HOMAIR en pacientes incidentes fue 1,8 ± 1,3 y 2,2 ± 2,1 en situación basal y a los 12 meses, respectivamente (no significativa [ns]). En pacientes prevalentes estos valores fueron 2,3 ± 1,3 y 2,5 ± 2,2 (ns). En nuestro estudio, las concentraciones medias de glucosa, insulina y RI medida por el HOMAIR y QUICKI (índice cuantitativo de control de la sensibilidad a la insulina) fueron similares en situación basal y al año de seguimiento, tanto en incidentes como en prevalentes. No objetivamos diferencias significativas en relación con la comorbilidad CV, cardiopatía isquémica, insuficiencia cardíaca o comorbilidad vascular cerebral o periférica, ni en función del índice HOMAIR, ni en el de los niveles de insulina. En conclusión, los pacientes no diabéticos en DP no presentan elevación significativa de los niveles de HOMAIR, ni se modifica con paso del tiempo en diálisis, lo que sugiere que la DP no es un factor de riesgo de RI. El hecho de que los índices de RI no se asocien a morbilidad o mortalidad CV parece sugerir el menor peso de este factor en el ámbito de la DP.
End-stage renal disease patients show early insulin resistance (IR), characterised by alterations in the carbohydrate metabolism and hyperinsulinaemia generally associated with dyslipidaemia and a proinflammatory condition. Cardiovascular disease (CVD) is the main cause of mortality in patients on dialysis. There is a strong association between IR, hyperinsulinism and CV disease. The objective of this study was to evaluate the effect of peritoneal dialysis (PD) on IR and its effects on the subsequent CVD morbidity and mortality in nondiabetic uraemic patients. It involved 69 nondiabetic patients on PD, 35 incident patients (≤ 3 months on PD) and 34 prevalent patients (> 3 months on PD), with 2 estimated insulin resistance measurements 12 months apart using the insulin resistance index (HOMAIR). The mean HOMAIR value in incident patients was 1.8 ± 1.3 and 2.2 ± 2.1 at baseline situation and at 12 months respectively (not significant [NS]). In prevalent patients these values were 2.3 ± 1.3 and 2.5 ± 2.2 (NS). In our study, the mean glucose, insulin and IR concentrations measured by the HOMAIR and QUICKI indexes (the latter being a quantitative control for insulin sensitivity control) were similar at baseline situation and the following year, in both incident and prevalent patients. We did not find any significant differences in relation to CVD comorbidity, ischaemic heart disease, heart failure or cerebrovascular or peripheral comorbidity neither in the HOMAIR index or insulin levels. To conclude, nondiabetic patients on PD do not display a significant increase in HOMAIR levels and this remains the case over time when on dialysis. This, in turn, suggests that PD is not an IR risk factor. The fact that the IR indexes are not associated with CVD morbidity or mortality seems to suggest that this is a less significant factor in the field of PD.
Cardiovascular disease (CVD) is the primary cause of death in patients on dialysis.1,2 The association between peripheral insulin resistance index (IR), hyperinsulinism, and CVD has been explored through numerous cross-sectional and prospective studies.1-4 In fact, hyperinsulinism is considered to be a good marker for IR in patients with significant hyperglycaemia. In addition, IR is associated with several risk factors for CVD, such as dyslipidaemia,2 arterial hypertension,5 and hypercoagulability.2
Patients with end-stage chronic kidney disease (CKD) develop early IR, characterised by altered carbohydrate metabolism with hyperinsulinemia, which is generally associated with dyslipidaemia and a pro-inflammatory pattern with altered serum levels of adipocytokines.6 Several studies have demonstrated that IR is possibly associated with a silent and systemic inflammatory process and a micro-inflammatory process characterised by the activation of peripheral cells such as polymorphonuclear cells and monocytes.7 The final consequence is an elevated rate of atherosclerosis and a high rate of morbidity/mortality due to CVD.8
Our working hypothesis was based on the possibility that glucose in peritoneal dialysis (PD) solutions could increase IR through an adipocytokine-mediated effect, and contribute to accelerating the process of atherosclerosis in uraemic patients. With this in mind, the objectives of our study included:
PATIENTS AND METHOD
Patients
Ours was a prospective and observational study involving a cohort of incident and prevalent patients treated with PD. A total of 69 nondiabetic patients on PD were included, 35 of which were incident (≤3 months on PD) and 34 of which were prevalent (>3 months on PD). In addition, 58% were treated with automated peritoneal dialysis, and 42% with continuous ambulatory peritoneal dialysis, with 2 measurements of insulin resistance taken 12 months apart using the homeostasis model assessment insulin resistance (HOMAIR). The purpose of this combined evaluation was to test the reproducibility, potential changes derived from PD, and the influence of these parameters on the appearance of CV events and mortality.
We excluded patients with active forms of cancer, acute infections, uncontrolled or systemic chronic inflammatory disease, or an initial glycaemia value >140mg/dl.
The causes of kidney disease were glomerulonephritis in 21 patients (30.4%), nephroangiosclerosis/vascular in 8 patients (11.6%), other causes in 8 patients (11.6%), polycystic kidney disease in 7 patients (10.1%), systemic disease in 7 patients (10.1%), unknown in 7 patients (10.1%), chronic pyelonephritis in 5 patients (7.2%), interstitial nephropathy in 5 patients (7.2%), and hereditary in 1 patient (1.4%).
At the time of the first measurement of IR, we considered baseline and start of follow-up times for the survival analysis.
Comorbidity was evaluated using the Charlson index as modified by Beddhu.9
LABORATORY PROCEDURES
Laboratory parameters were measured at baseline values and after 1 year of evolution, always following 12 hours of fast and maintaining normal regimens of PD treatment. We also compiled dialysis parameters, such as Kt/V from weekly urine samples and normalised protein nitrogen appearance (nPNA).
Insulin was measured using a double enzyme immunometric assay method with an AIA 360 auto-analyser (Tosoh Bioscience). The inter and intra-trial coefficients of variation were 1.7% and 3.3%, respectively. The sensitivity of the analysis was 1µU/ml. The normal range for insulin levels in our laboratory was 2-17µU/ml.
Based on baseline insulinemia and glycaemia values, we calculated HOMAIR index scores based on the formula by Matthews et al.10: HOMAIR = glucose (mmol/l) x insulin (µU/ml)/22.5. We also calculated the quantitative insulin sensitivity check index based on the protocol by Hrebicek et al.11: QUICKI = 1/[log fasting insulin level (mU/l) – log fasting glycaemia (mg/dl)].
Definitions
Coronary disease: a history of angina, myocardial infarction, coronary angiography evidence, percutaneous intervention, or coronary bypass.
Congestive heart failure: New York Heart Association (NYHA) classification12:
- Functional class I: normal activity with no symptoms. No limitations to physical activity.
- Functional class II: the patient tolerates normal activity, but physical activity is slightly limited due to dyspnoea that appears during intense exercise.
- Functional class III: the patient’s capacity for physical activity is lower than normal, with notable limitations due to dyspnoea.
- Functional class IV: the patient suffers dyspnoea upon the slightest effort or even at rest, and is incapable of performing virtually any physical activity
Peripheral arterial disease: patients with intermittent claudication, arterial bypass, amputation, gangrene, acute arterial insufficiency, or unrepaired aneurysms of the thoracic or abdominal arteries greater than 5cm.
Cerebral vascular disease: patients with a history of cerebrovascular accidents or transitory ischaemic accidents (with mild or zero sequelae).
Dyslipidemia: one or more of the following criteria13: 1) LDL (low-density lipoprotein) cholesterol ≥100mg/dl; 2) non-HDL (non-high-density lipoprotein) cholesterol ≥130mg/dl; 3) triglycerides ≥150mg/dl; 4) HDL cholesterol ≤40mg/dl; and 5) the use of one or more lipid-lowering drugs.
Hypertension: blood pressure (BP) was measured using a digital monitor (HEM-907) with cuffs adapted to the circumference of each patient’s arm, once the patient had been laying at rest for at least 5 minutes. Hypertension was diagnosed when the patient fulfilled one or more of the following criteria14: 1) systolic BP≥135mm Hg; 2) diastolic BP≥85mm Hg; and 3) the use of one or more anti-hypertensive drugs.
STATISTICAL ANALYSIS
Values were expressed as means (± standard deviation) and percentages. Proportions were compared using chi-square tests, and means were compared using Student’s t-tests for variables with a normal distribution, and Mann-Whitney or Wilcoxon tests were used for non-parametric quantitative variables. We performed a linear regression analysis using Pearson and Spearman correlation coefficients. We used the Kaplan-Meier method for the survival analysis, and compared curves using the log-rank method. A P-value <.05 was considered to be statistically significant for all cases.
RESULTS
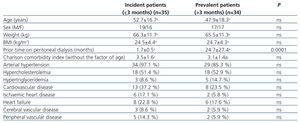
Table 1 summarises the baseline clinical characteristics for the two patient groups, classified based on prior time on PD (incident and prevalent patients). We did not observe significant differences between the two groups.
The mean time on PD was 32.1±16.0 months (range: 11-87 months).
Table 2 displays the mean dialysis parameters, laboratory values, and insulin resistance indices (HOMAIR and QUICKI) at initial evaluations and after 12 months of follow-up. We only observed statistically significant differences between the two groups in the fields of residual renal function, total cholesterol, and LDL cholesterol.
At the start of treatment, 60% of incident patients were on treatment with icodextrin, as compared to 57.1% after 12 months. In prevalent patients, the rate of use of icodextrin was 73.5% at both evaluations.
HOMAIR and initial insulin levels
When patients were classified based on median initial HOMAIR score (HOMAIR: 1.7; range: 6.29), we observed that incident patients with higher HOMAIR scores had significantly higher levels of total calcium and ionised calcium as compared to patients with lower HOMAIR scores. In prevalent patients, we did not observe significant differences for this parameter (Table 3). Upon classifying patients based on median insulinemia (7.0; range: 2-25), the findings were similar.
We did not observe differences in HOMAIR or QUICKI scores based on the different concentrations of glucose in peritoneal dialysate solutions, both at the start of the study and after 1 year (data not shown).
Comorbidity analysis
When we analysed patients based on the median value for baseline HOMAIR score, we did not observe statistically significant differences for CV comorbidity, ischaemic heart disease, heart failure, or cerebral/peripheral vascular comorbidity. We encountered the same result when separating patients based on the median value for initial insulin.
We did not observe a significant difference between patients with HOMAIR scores above and below the median value in terms of Charlson comorbidity score (with age excluded) (3.2±1.6 vs. 3.4±1.3).
The presence of CVD did not significantly affect HOMAIR scores.
Survival analysis
Eighteen patients (26%) died during the follow-up period, with death of vascular origin being the most common source of mortality (n=5; 27.8%), followed by cardiac causes (n=4; 22.2%), infectious causes (n=4; 22.2%), deterioration (n=2; 11.1%), tumours (n=1.5; 6%) and other (n=2; 11.1%).
Survival analysis revealed that patients who died were mostly male (11 vs 7; P=.001), elderly (63.6±12.3 vs 45.7±16.8 years of age; P=.000), and a higher Charlson index score (with age excluded) (4.5±1.1 vs 2.9±1.3; P=.000). We did not observe statistically significant differences in terms of IR index values (data not shown).
The global Kaplan-Meier survival analysis did not reveal significant differences in terms of mortality between patients with HOMAIR index scores above and below the median value (Figure). In a similar manner, we did not observe differences in terms of CV mortality and fatal/non-fatal CV events during the study (data not shown).
DISCUSSION
In this study, we analysed the effects of PD on parameters of insulin resistance (HOMAIR and QUICKI) and the influence of these scores on the appearance of CV events and mortality in a group of nondiabetic PD patients.
IR is very common among uraemic patients, occurring even in early phases.15 Several different hypotheses have been proposed in an attempt to explain IR: 1) hyper-production of endogenous proinflammatory factors such as homocysteine,16 oxidative stress,17 and elevated lipoprotein (a) levels,18 and exogenous proinflammatory factors such as chronic infections (sometimes silent) by Helicobacter pylori,19 infected vascular prostheses, or intravenous iron administration, among others20; and 2) increased adipose tissue21 due to excess caloric intake, which leads to a vicious circle with increased production of adipocytokines (leptin, resistin) and other proinflammatory mediators such as nuclear factor kappa beta, tumour necrosis factor alpha, and interleukin 6.22 These chemokines act as chemotactic proteins for monocytes and macrophages (MCP-1),23,24 thus perpetuating IR.
In patients on PD, the development of IR is attributed to the excessive amount of glucose absorbed through dialysate fluids, which contributes to other metabolic disorders such as central obesity, hypertriglyceridemia, and de novo diabetes.25 This process also induces hyper-secretion of adipocytokines, which perpetuate hyperinsulinism and its deleterious metabolic and systemic effects. According to the study by Fortes et al.,26 patients on PD have higher fasting glucose levels, HbA1c, and HOMAIR index scores than patients on haemodialysis. Also, patients who receive dialysis with glucose-free dialysate exhibit a lower absorption of glucose, lower weight gain and fat accumulation, reduced levels of plasma leptin and increased levels of plasma adiponectin, and improved IR and dyslipidemia.27,28 In our study, mean glucose, insulin, and IR (as measured by HOMAIR and QUICKI indices) levels were similar between initial measurements and after 1 year in both incident and prevalent patients.
Bonora et al.29 examined a large sample of patients with type 2 diabetes, and showed that IR (as estimated using the HOMAIR index) was a strong predictor for CVD, both at the start of the study and during follow-up. This correlation was independent of traditional CV factors and variables closely related to IR, such as body mass index. Also, despite the fact that several studies have shown that hyperinsulinemia is capable of predicting CVD,4,30 others, in contrast, have not found a significant association between plasma insulin levels and CVD.31 In our study, we did not observe significant differences in terms of CV comorbidity, ischaemic heart disease, heart failure, or cerebral vascular comorbidity based on HOMAIR index scores or insulin levels. The Charlson index score (without the factor of age included) was also similar among all patients. Nor did we observe a relationship between the presence of prior CVD and HOMAIR scores.
Glucose in PD solutions may have an effect on abdominal adipocytes, which, through an increase in the secretion of adipocytokines, may increase IR, thus favouring the development of metabolic syndromes and accelerating the process of atherosclerosis in uremic patients. Upon analysis of our results, we did not observe significant differences in HOMAIR or QUICKI index scores based on the concentrations of glucose used in PD solutions.
In the general population, IR is considered to be a factor for CV risk and mortality,32,33 whereas this factor appears to have a different significance in studies carried out with CKD patients. A study by Shinohara et al.34 demonstrated that HOMAIR scores predicted mortality in nondiabetic patients with CKD. However, other studies have not observed this relationship.35 In our survival analysis, we did not observe statistically significant differences in terms of IR scores or when classifying patients based on median HOMAIR values in a Kaplan-Meier analysis. It may be that other characteristics of these patients, such as age or comorbidity, may play a greater role in determining survival than insulin resistance. In addition, the HOMAIR scores recorded in our study fell within normal limits, which would also impede the possibility of significant differences in terms of survival.
Bonora et al.36 observed mean HOMAIR values of 2.06±0.14 in a population of 62 healthy individuals using a double-antibody radioimmunoassay for measuring plasma insulin. In a study involving 490 nondiabetic volunteers, 77% of which were Caucasian, of both sexes, aged 19-79 years, and with a body mass index of 18-42.2, the researchers observed a mean HOMAIR score of 2.7±.1.37 The mean HOMAIR value in normal individuals (with no glucose metabolism disorders) from a study prior to ours was 2.22±0.26.38 The HOMAIR values recorded in our study, both in incident and prevalent patients, placed between the 50th and 75th percentile of the values recorded for the general population in Spain according to a recent study.39 Caravaca et al.40 observed higher mean HOMAIR values than ours (mean: 4.28±2.07) in a population of nondiabetic patients with CKD. These data support the results obtained in nondiabetic patients on PD, indicating that HOMAIR scores are not significantly elevated in these individuals as compared to the general population, and that this result does not vary over time on dialysis, at least within a period of one year.
We recognise that our study involves the limitation of only including patients with an initial glycaemia >140mg/dl instead of the cut-off value for diagnosing diabetes in the general population of ≥126mg/dl, but since our study involved patients on PD with a continuous glucose input, we intended to assess a cohort of patients with a baseline glucose level close to normal values, although with the exclusion of diabetic patients. It is possible that, through the use of this more permissive threshold, we may have included patients with less severe disorders of glucose metabolism, such as carbohydrate intolerance, but it was our intention to only exclude patients with clear cases of diabetes. The fact that, in order to be included in our study, all patients needed to have at least one year on PD also implies a bias in our survival analysis. In addition, this study is limited by the small number of patients evaluated, but it does offer a double approach assessing patients at different stages of PD treatment (initial and late), with consistent results across both groups. The prospective approach used also considerably bolsters the results obtained.
To conclude, nondiabetic patients on PD do not have significantly elevated HOMAIR scores, or modifications to these values after one year of treatment on PF, which suggests that PD is not the cause of increased risk of IR. The fact that IR indices were not associated with CV morbidity/mortality rates appears to suggest that this factor holds little sway in the field of PD.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline characteristics of incident and prevalent patients on peritoneal dialysis
Table 2. Dialysis parameters, laboratory values, and peripheral insulin resistance indices in patients on peritoneal dialysis; baseline and after 1 year of follow-up
Table 3. Comparison of baseline values for laboratory parameters based on median HOMAIR values (median: 1.7; range: 6-29)
Figure 1. Kaplan-Meier curves, median HOMAIR vs patient death