Introducción: El déficit de 25-hidroxivitamina D (25OHD) asociado a un hiperparatiroidismo secundario son hallazgos frecuentes en pacientes con enfermedad renal crónica (ERC) en hemodiálisis (HD). Estos hechos se asocian con un incremento de la morbimortalidad de origen cardiovascular (CV). Niveles séricos adecuados de 25OHD, así como el uso de activadores selectivos del receptor de vitamina D (AsRVD), han demostrado tener efectos beneficiosos sobre el metabolismo óseo-mineral y el riesgo CV de manera independiente. Actualmente aún existe controversia respecto al tipo de suplementación que precisan los pacientes con ERC en HD. Objetivo: El objetivo de nuestro estudio fue evaluar si existe beneficio alguno en el tratamiento combinado de 25OHD, calcifediol oral y AsRVD, paricalcitol oral sobre el metabolismo óseo-mineral y marcadores inflamatorios, respecto al tratamiento único con cada uno de ellos, en un grupo de pacientes de HD. Material y métodos: Realizamos un estudio prospectivo de 6 meses de duración sobre 26 pacientes de nuestra unidad en HD. Aleatorizamos a los pacientes en dos grupos; el grupo 1 (G1) recibió tratamiento con paricalcitol oral a dosis de 1 μg/día. El grupo 2 (G2) fue tratado con calcifediol 1 ampolla/sem (0,266 mg/sem = 16.000 U) por vía oral. Trascurridos 3 meses de tratamiento, al G1 se le añadió calcifediol y al G2, paricalcitol a las mismas dosis, manteniendo dichos tratamientos durante 3 meses más, hasta completar los 6 meses de seguimiento. Las determinaciones analíticas se llevaron a cabo en los meses 0, 3 y 6, midiéndose en todos los pacientes los marcadores séricos de 25OHD, calcio, fósforo y hormona paratiroidea (PTH); como marcadores de remodelado óseo se midió la fosfatasa alcalina, propéptido aminoterminal del procolágeno tipo 1 (Pinp1) y el telopéptido carboxilo-terminal del colágeno tipo I (Cross Laps); marcadores inflamatorios (interleuquina 8 [IL-8]). Asimismo se recogieron datos de niveles de insulina, glucosa, hemoglobina, agentes eritropoyéticos (AEE) e índices de resistencia a la eritropoyetina y HOMA (homeostasis model assessment). Resultados: Se detecta un déficit de 25OHD en todos los pacientes a estudio, con una media de 13,67 ± 4,81 ng/ml. La suplementación con calcifediol oral aislado corrige este déficit sin evidencia de toxicidad (35,36 ± 33,68 ng/ml en el G1 a los 6 meses y 59,21 ± 26,50 ng/ml en el G2 a los 3 meses). El tratamiento con paricalcitol reduce de forma significativa los niveles de PTH en el G1 a los 3 meses (p < 0,039) no observándose esta significación, aunque sí descenso de la PTH, en el G2 tras su introducción a partir del tercer mes. Asimismo, observamos una disminución del marcador óseo Pinp1, con paricalcitol sin otros cambios, apuntando a un posible efecto directo sobre las células óseas (p < 0,001). Tanto el tratamiento con calcifediol como con paricalcitol producen una significativa disminución en los niveles de IL-8 (p < 0,001), conocido marcador inflamatorio, llamando la atención una tendencia a mejor respuesta a los AEE, en posible relación con este descenso de la inflamación. El índice HOMA no cambió de forma significativa. Conclusión: Con nuestros resultados, no podemos concluir que la asociación calcifediol-paricalcitol produzca ventajas sobre el efecto de cada uno de ellos por separado en los marcadores medidos. Paricalcitol además, por sí solo, parece tener efecto directo sobre la remodelación ósea.
Background: The deficit of 25-hydroxyvitamin D (25OHD) associated with secondary hyperparathyroidism (SHPT) is a frequent finding in chronic kidney disease (CKD) patients on haemodialysis (HD). These events are associated with increased morbidity and mortality rates of cardiovascular (CV) origin. Adequate 25OHD serum levels as well as the use of selective vitamin D receptor activators (VDRA) have been shown to have beneficial and independent effects on bone mineral metabolism and cardiovascular risk. Currently, there is still controversy regarding the type of supplementation needed by CKD patients on HD. Objective: The aim of our study was to evaluate whether there is a benefit of combination therapy with 25OHD, calcifediol and a VDRA, oral paricalcitol, on bone-mineral metabolism and inflammatory markers, compared to single treatment with each of these in a group of patients on HD. Material and method: We performed a prospective study of 6 months, involving 26 patients in our HD unit. We randomised patients into two groups: group 1 (G1) received oral paricalcitol treatment at doses of 1mcg/day. Group 2 (G2) was treated with 1 ampoule calcifediol/wk (0.266mg/wk=16 000U) orally. After 3 months of treatment, calcifediol and paricalcitol were added to the G1 and G2 respectively at the same doses, keeping these treatments together for 3 months to complete the 6 months of follow-up. Laboratory tests were performed at months 0, 3 and 6, measuring in all patients serum markers of 25OHD, calcium (Ca), phosphorus (P) and PTH. Bone turnover markers measured were: alkaline phosphatase (AP), aminoterminal propeptide of procollagen type 1 (Pinp1) and carboxyl-terminal telopeptide of type I collagen (CrossLaps), and inflammatory markers: IL-8. We also collected data on levels of insulin, glucose, haemoglobin, erythropoiesis-stimulating agents (ESAs) and rates of resistance to EPO and HOMA (homeostasis model assessment). Results: We detected a deficit of 25-hydroxyvitamin D in all patients studied, with a mean of 13.67±4.81ng/ml. Supplementation with oral calcifediol significantly corrects this deficit without evidence of toxicity (35.36±33.68ng/ml in G1 at 6 months and 59.21±26.50ng/ml in G2 at 3 months). Paricalcitol treatment significantly reduces PTH levels in G1 at 3 months (P<.039). We also noted a decrease in bone marker Pinp1 with paricalcitol, pointing to a possible direct effect on bone cells (P<.001). Both treatment with paricalcitol and with calcifediol produced a significant decrease in levels of IL-8 (P<.001), a known inflammatory marker, drawing attention to a trend towards better response to erythropoiesis-stimulating agents (ESAs), possibly related to the decrease in inflammation. The HOMA index did not change significantly. Conclusion: Based on our results, we cannot conclude that the association of calcifediol and paricalcitol produces advantages over the effect of each drug separately. In addition, Paricalcitol by itself appears to have a direct effect on cellular bone remodelling.
INTRODUCTION
There is a growing tendency for the use of vitamin D, both in the general population and in patients on haemodialysis (HD), because of the potential pleiotropic benefits that go beyond the activity of vitamin D on bone-mineral metabolism.1-4
Low serum values of 25-hydroxyvitamin D (25OHD) have been associated with a greater mortality rate among incident patients on HD.5-6 Current guidelines published by the Spanish Society of Nephrology (S.E.N.) for the management of bone-mineral metabolism alterations in chronic kidney disease patients (S.E.N.-MM) recommend measuring vitamin D levels (calcidiol) in order to prevent and treat the common insufficiency/deficiency of this molecule. Serum calcidiol values <30ng/l are considered to constitute “insufficiency”, and <15ng/l is a “deficiency”. However, there is a lack of information from studies in the general population to demonstrate that values greater than 40ng/l have any added benefit.7
Selective activation of vitamin D receptors with paricalcitol has also been proven to be beneficial not only in the control of secondary hyperparathyroidism (SHPT), but also in reducing inflammation and cardiovascular risk.1,2,8-12
Very few studies have examined the effects of combined therapies involving supplements of vitamin D and selective activation of vitamin D receptors, but there appears to be an additive beneficial effect.13-15 With the present study, we intended to evaluate the possible benefits of combined treatment of 25OHD or calcifediol and a selective activator of the vitamin D receptor, paricalcitol, on bone-mineral metabolism and inflammatory markers, as compared to treatment with each strategy individually, in a group of patients on HD treated at our hospital.
MATERIAL AND METHOD
Ours was a prospective study involving 26 chronic kidney disease patients treated with HD at our hospital during 2011. We randomised patients into two treatment groups: group 1 (G1) included 11 patients and group 2 (G2) included 15. G1 received treatment with oral paricalcitol at 1μg/day. G2 was treated with oral calcifediol at 1 ampoule/week (0.266mg/week = 16 000U). After 3 months had passed, the treatment regimen for G1 was supplemented with calcifediol, and paricalcitol was added to the treatment for G2 at the same doses as prescribed during the first three months, maintaining these new treatment regimens for another 3 months until reaching a total of 6 months of follow-up. The calcium concentration in dialysate solutions was the same for all patients, at 1.25mmol/l (2.5mEq/l; 5mg/dl). Prior to the study, 2 patients in G1 were on treatment with vitamin D for 14 months, and 4 patients in this group were on this treatment for 16 months. These patients went through a wash-out period of 1 month prior to inclusion in the study. Laboratory analyses were carried out at 0, 3, and 6 months.
Patients
Of the 26 patients studied, 77% were male, with a mean age of 62.3±10.1 years. In G1, 72% of patients were male, with a mean age of 73.6±9.8 years, vs. 73% in G2 with a mean age of 67.9±13.5 years. All patients were on chronic HD programmes, with sessions 3 times per week at 4 hours/day. In G1 and G2, 54% and 66% of patients, respectively, were on treatment with calcium-based binders, and 46% and 34% of patients in these groups, respectively were on treatment with non-calcium binders. Throughout the study, the doses of these binders were not modified. Only one patient in G1 and three in G2 took calcimimetics. Nine patients in G1 (81%) were treated with anti-hypertensives, as compared to 12 patients (80%) in G2. As regards erythropoiesis-stimulating agents (ESA), 81% of patients in G1 and 73% in G2 were under treatment. No patients became unstable based on clinical and/or laboratory parameters during the 6 months prior to inclusion in the study, and no events occurred during the follow-up period that might alter study results. As inclusion criteria, we evaluated parathyroid hormone (PTH) >300ng/ml, calcium <10mg/dl, and no criteria for phosphorous levels were taken into account. Both groups included diabetic patients (G1: 1 patient; G2: 2 patients), and no exclusion criteria were established except for the aforementioned clinical/laboratory stability.
Laboratory analyses
Sample extraction
Blood samples were taken systematically from all patients under fast between 08:00 and 09:00 in the morning. We extracted 10ml of blood from each patient in silicon vacuum tubes with silica gel filters and no anticoagulants for serum samples, as well as a 5ml sample in an ethylenediaminetetraacetic acid (EDTA) tube (1mg/ml) for plasma samples. We used Vacutainer® (Becton-Dickinson, Meylan, Cedex-France) vacuum tubes.
All samples were processed before one hour had passed since extraction. Serum tubes left to coagulate for 20-30 minutes and then centrifuged at 2000g at room temperature. Plasma from the EDTA tubes was aliquoted into Eppendorf® tubes, marked, and frozen at –80ºC for later processing.
Biochemical parameters
We measured serum levels of 25OHD for all patients using automated chemiluminescent specific immunoassays in an iSYS® (IDS-iSYS Multi-Discipline Automated Analyser, Pouilly-en Auxois, France); we extracted the 25OHD metabolite with acetonitrile in the machine, and then detected free vitamin D by chemiluminescence. Sensitivity was at 5ng/ml with an intra-assay reproducibility <10%, and inter-assay reproducibility <15%. Normal serum vitamin D levels were established as 20-60ng/ml. The latest American guidelines set vitamin D insufficiency at <20ng/ml.
Serum phosphorous and calcium were measured automatically in an ADVIA 1650 Analyser® (Siemens HealthCare Diagnostics, Mannheim, Germany), with normal ranges set at 8.1-10.7mg/dl and 2.7-4.5mg/dl, respectively.
We measured PTH using an automated immunological sandwich assay using a Liaison analyser by DiaSorin® (Deutschland, Dietzenbach), with 2 specific monoclonal antibodies: one for the 39-84 fragment, and another for the 1-34 amine-terminal fragment LIAISON® N-TACT® PTH Assay, DiaSorin). This assay recognises intact 1-84 PTH, but cross-reacts with the recently identified 7-84 fragment (of unknown clinical significance). The sensitivity for this test was 1pg/ml. Intra- and inter-assay reproducibility for this test as 2.6% and 5.8%, respectively. Normal values for our population were <45pg/ml.
Haemoglobin was quantified using a Gen-S Analyser® (Beckman Coulter TM, Hialeah, FL USA) and a Coulter S+ Counter® (Coulter, Hialeah, FL USA). Insulin was measured using an automated immunological sandwich assay with a Liaison analyser by DiaSorin® (Deutschland, Dietzenbach) using 2 specific monoclonal antibodies. The sensitivity of this test was 0.2mU/ml. Intra- and inter-assay variation coefficients for this technique were below 4% and 10%, respectively, and the normal range for this variable was established at 2-17mU/ml. Glucose was measured in the same manner as serum calcium and phosphorous.
We also took into account erythropoiesis-stimulating agents, rates of resistance to erythropoiesis-stimulating agents (ESA/Hb), and HOMA index (homeostasis model assessment) as a marker for insulin resistance.
Bone remodelling markers
Bone alkaline phosphatase was determined using an enzyme immunoassay (EIA®) (Alkphase B kit, Metra Biosystems, Mountain View, CA, USA). Sensitivity for this test was 0.7U/l. Intra- and inter-assay variations were 3.5% and 6.2%, respectively. Specificity: bone: 100%; hepatic: 3%-8%; placental: 0%; and intestinal: 0.4%. Normal values: 12-23U/l.
We analysed Pinp1 using an automated chemiluminescent specific immunoassay with an iSYS® (IDS-iSYS® Multi-Discipline Automated Analyser, Pouilly-en Auxois, France); the sensitivity for this test was 2µg/l and the intra- and inter-assay reproducibility was <5% and <8%, respectively.
CrossLaps were evaluated using ELISA (Nordic Bioscience Diagnostics, Herlev Hovedgade, Demark). The sensitivity for this test was 0.010ng/ml; intra- and inter-assay variability was 5.1% and 6.6%, respectively. Ranges of normality were 0.142-0.522ng/ml in males, 0.166-0.567ng/ml in premenopausal females, and 0.251-0.761ng/ml in post-menopausal females, although this last range of values is more debatable.
Inflammation marker
We measured serum interleukin 8 (IL-8) levels in all patients as an inflammatory marker; we performed this analysis using an R&D Systems® kit (Minneapolis, MN, USA).
Statistical analysis
We performed all statistical analyses using IBM SPSS® software, version 19. We performed a descriptive and comparative analysis between drug treatments for the study variables, examining means and standard deviation. We evaluated the possible statistical differences between the drug treatments in terms of interval and time, and also examined the evolution of each drug; we performed Student’s t-tests. The cut-off for statistical significance was set at P<.05.
RESULTS
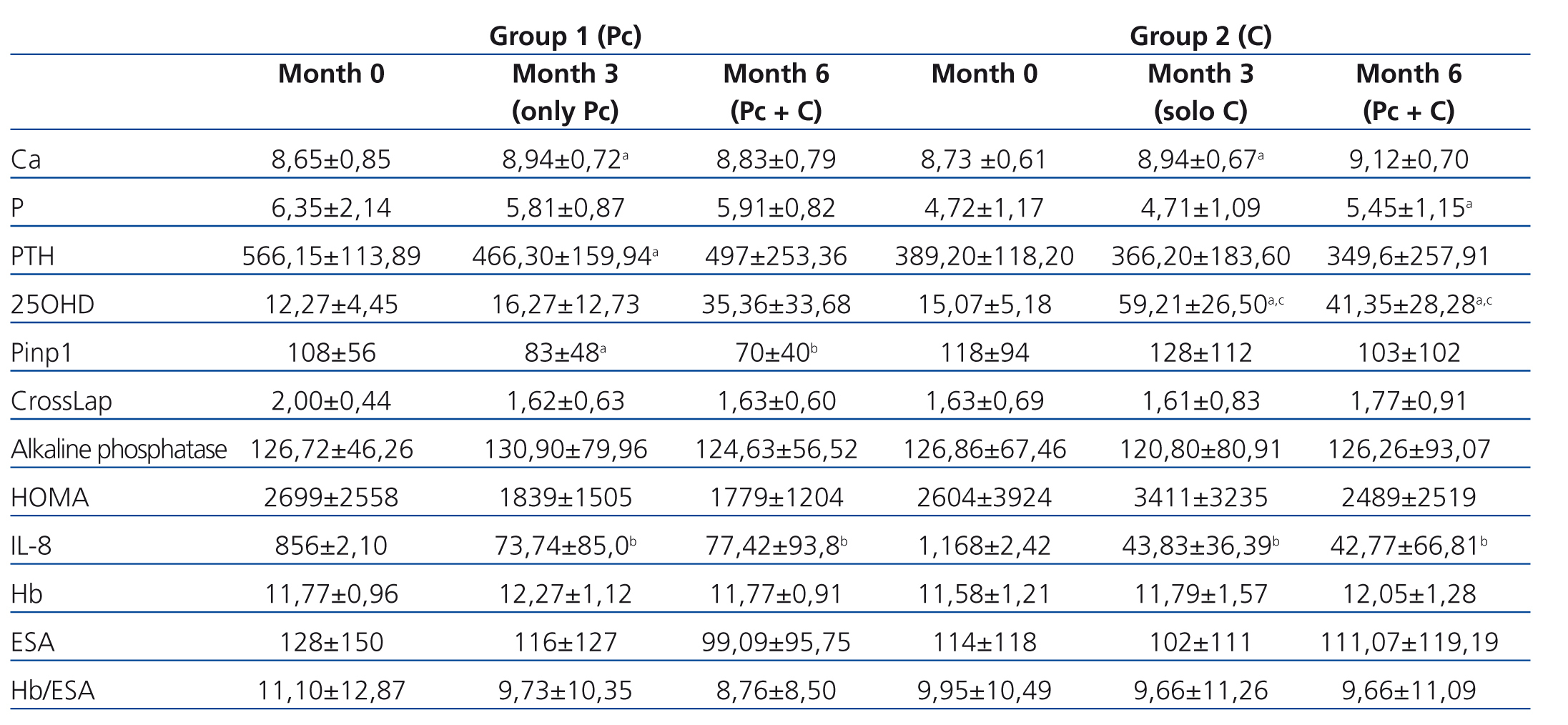
We observed a marked deficit in serum 25OHD levels in study patients, both in G1 and G2, with a mean of 13.67±4.81ng/ml at the start of the study (G1: 12.27±4.45ng/ml, range: 10.50-21.34ng/ml; G2: 15.07±5.18ng/ml, range: 9.45-24.90ng/dl). Using calcifediol supplements corrected this deficit. In G1 patients treated with paricalcitol until 3 months, initial 25OHD levels were 12.27±4.45ng/ml, with an increase after 3 months to 16.27±12.73ng/ml, a non-significant difference. After adding oral calcifediol to the treatment regimen for this group for the following 3 months at doses of 16 000U/week, 25OHD levels were a mean 35.36±33.68ng/ml. In G2, in which patients were initially treated with calcifediol, mean 25OHD levels were 15.07±5.18ng/ml at the start of the study, and increased to 59.21±26.50ng/ml after three months of treatment (P<.05). We did not observe a continued increase in 25OHD levels in this group after adding paricalcitol after the third month (41.35±28.28ng/ml; non-significant P-value). We did not observe any cases of toxicity or adverse effects from administering vitamin D supplements.
G1 patients exhibited significantly higher baseline PTH values than G2 patients (566.15±113.89pg/ml vs. 389.20pg/ml; P<.003). In G1 patients, who received paricalcitol from the beginning of the study until three months, we observed a significant decrease in PTH values (566.15±113.89pg/ml [initial] vs. 466.30±159.94pg/ml [three months]; P<.039). G2 patients, who were treated during this initial period with calcifediol, also exhibited a decrease in PTH levels, although this difference was not significant (389.20pg/ml [initial] vs. 366.60±183.60pg/ml [three months]; P<.607). After the third month, in which the treatment for G1 patients was supplemented with calcifediol, this decrease in PTH values stopped (466.30±159.94pg/ml after three months vs. 497.30±253.36pg/ml after six months). In G2, no changes were observed in PTH levels after adding paricalcitol after the third month (Table 1).
Upon comparing serum calcium levels, we observed an increase in both groups over the course of the study. In G1, initial calcium values were 8.65±0.85mg/dl, ending at 8.94±0.72mg/dl after three months (P<.003). Initial serum calcium values in G2 were 8.73±0.61mg/dl, ending at 8.94±0.67mg/dl after three months (P<.004). After 6 months, mean serum calcium values had increased in both groups as compared to third month values, although this increase was not significant (G1: 8.83±0.79mg/dl; P<.55, and G2: 9.12±0.70mg/dl; P<.15). Neither group exhibited increases in serum phosphorous levels after three months of treatment with the doses provided: G1: 6.35±2.14mg/dl; P<.039 initial vs. 5.81± 0.87mg/dl; P<0.39 after three months, and G2: 4.72±1.17mg/dl; P<.93 initial vs. 4,71±1.09mg/dl; P<.93 after three months; however, we did observe an increase in phosphorous levels after administering both drugs together (month six), with a significant difference in G2 (4.71±1.09 [third month] vs. 5.45±1.15mg/dl [sixth month]; P<.05), and a non-significant difference in G1 (5.81±0.87mg/dl [third month] vs. 5.91±0.82mg/dl [sixth month]; P<.98).
As regards bone remodelling markers, we assessed Pinp 1, CrossLaps, and alkaline phosphatase (AP). We observed a tendency towards decreases in bone markers in the paricalcitol group. In G1, we observed a decrease in Pinp 1 from 108±56μg/l (initial) to 70±40μg/l (end of follow-up); P<.001. We observed a non-significant decrease in CrossLaps and in AP. In G2, in which patients were initially treated with calcifediol, we observed a non-significant decrease in Pinp, with no changes in Cross Laps (1.63±0.69pg/ml [initial] vs. 1.77±0.91pg/ml) or in AP (126.86±67.46U/l [initial] vs. 126.26±93.07U/l) (Table 1).
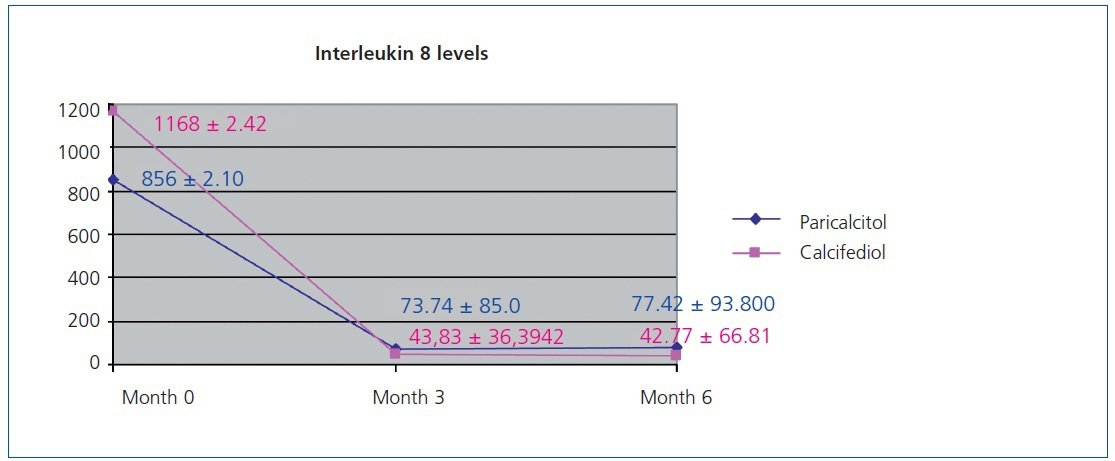
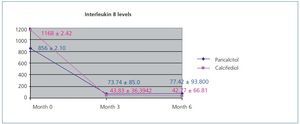
Figure 1 displays the very significant (P<.0001) decrease with both paricalcitol and calcifediol treatments in the inflammatory marker IL-8, a known biomarker for cardiovascular risk. The combination of both drugs did not produce a more marked decrease than each drug alone.
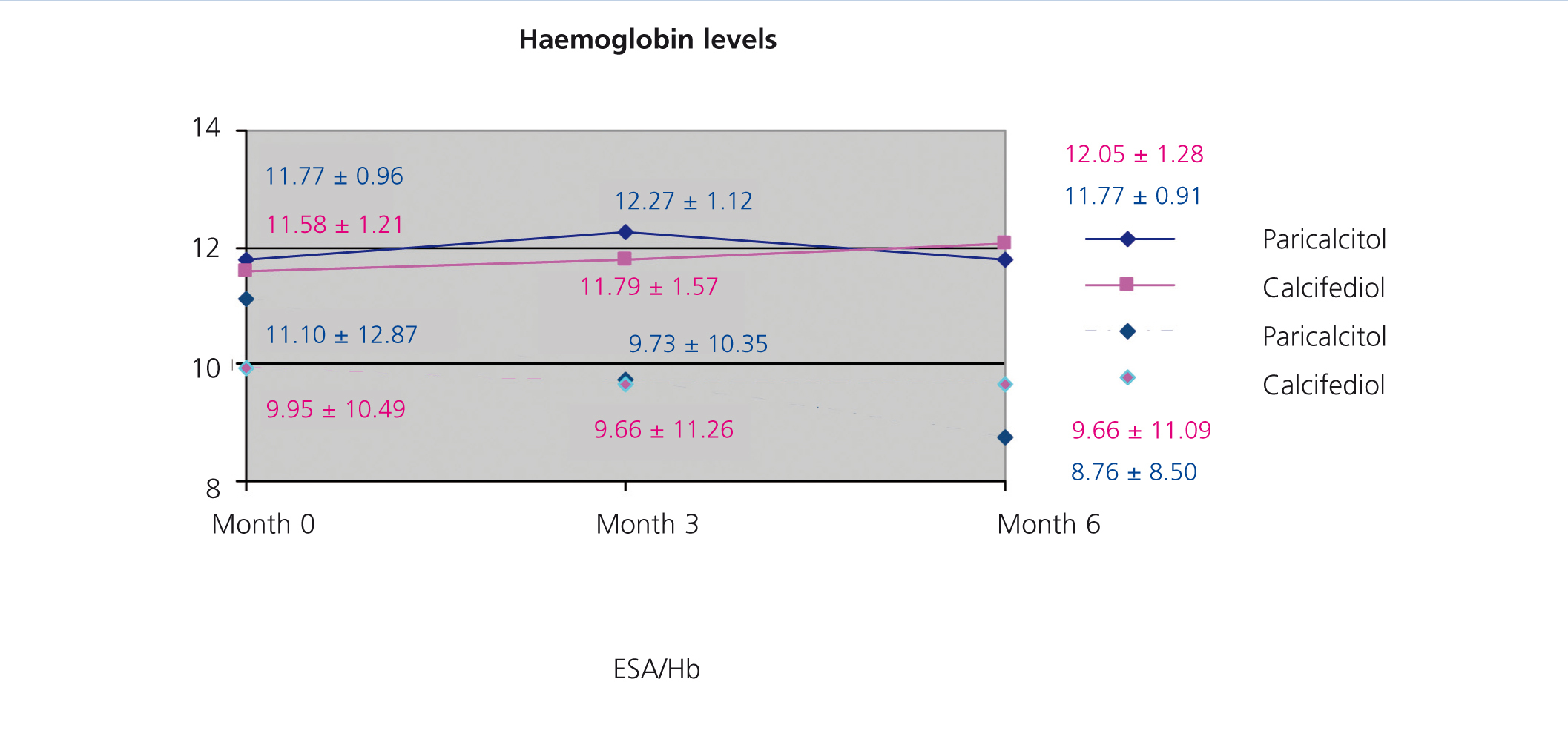
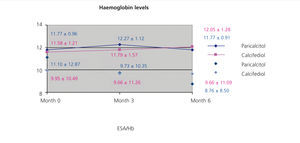
We did not observe significant differences when treating patients with paricalcitol, calcifediol, or both in combination in terms of haemoglobin values or erythropoietin (EPO) doses, although we did observe an improvement in sensitivity to EPO in the group of patients who started treatment with paricalcitol, with a decrease in resistance rates (figure 2). A larger study sample would probably have resulted in significant differences, perhaps due to improved levels of inflammatory markers.
The HOMA index allows for making estimations of insulin resistance and beta cell function by measuring plasma concentrations of insulin and plasma during fast.13-15 After analysing this variable, we observed a non-significant decrease in patients treated with paricalcitol; in contrast, this decrease was not observed in patients treated with calcifediol.
DISCUSSION
As has already been established, patients who undergo renal replacement therapy often have deficient blood levels of 25OHD. Among others, low dietary intake in addition to decreased intestinal absorption and uraemia all contribute to this deficit.13,14,16,17 The most recent S.E.N. recommendations for managing bone-mineral metabolism alterations in chronic kidney disease patients include measuring vitamin D (calcidiol) levels in order to prevent and treat deficiency/insufficiency, which is such a common phenomenon. Deficient values are those below 15ng/l, and insufficiency is defined as values <30ng/l, although no added benefits have been shown for values >40ng/l.
The results from the patients in our study reflect this situation. Our results indicated mean serum 25OHD values of 13.67±4.81ng/ml. The lack of this vitamin contributes to increased progression of SPTH18,19 and abnormalities in mineralisation, which may justify administering supplements, although no current consensus exists for providing supplements to patients on chronic dialysis treatment, many of which have vitamin D deficiencies. The patients in our study received 1 ampoule/week (16 000U/week) of oral calcifediol; in the G1, in which patients received calcifediol from the third to sixth months, patients reached adequate mean values (35.36±33.68ng/ml), and in the G2, in which patients received calcifediol from the start of the study to three months, reached levels of 59.21±26.50ng/ml. No patients suffered episodes of toxicity or any adverse effects from these supplements.
However, 25OHD remains as an inactive vitamin, requiring 25-hydroxylation and 1α-hydroxylation in the liver and kidneys, respectively, in order to be active, both of which are deficient in renal patients. This justifies providing active vitamin D supplements in our patients. Experimental studies have demonstrated that the combination of calcidiol and paricalcitol provides better anti-inflammatory and anti-fibrotic results.19 Low 25OHD values have been correlated with a greater degree of mortality in incident patients on HD, and the use of active vitamin D derivatives has been shown to resolve this issue.3-5,13,14,16
With this in mind, we performed this study in order to evaluate the possible effect of combined treatment with calcifediol and paricalcitol on bone-mineral metabolism and inflammatory parameters in patients on HD.
Our patients experienced a decrease in serum PTH levels after three months of treatment with paricalcitol, although, as in other studies, we observed an increase in serum calcium levels in both groups, this change being most notable in the G2 after incorporating paricalcitol. The decrease in serum PTH and P was more notable in G1 when starting treatment with paricalcitol than in G2, a difference that might be explained by the apparently more advanced state of SPTH in these patients. As such, it is difficult to conclude from our results a direct effect exerted by paricalcitol on controlling SPTH independently of calcium levels. However, based on previous publications and our results, we can say that in our patients, paricalcitol appears to reduce PTH levels. Excessively high P values were not produced in either group, although we did observe a slight increase in both groups after the third month of treatment.
Many studies have reported a correlation between chronic kidney disease and increased cardiovascular risk. Addabbo et al.20 demonstrated a tight relationship between increased intimal-medial thickness, the number of plaques, and the interior diameter of the carotid artery with respect to elevated levels of the inflammatory markers interleukin 6 (IL-6), matrix metalloproteinase-9 (MMP-9), plasminogen activator inhibitor-1 (tPAI), and vascular endothelial growth factor (VEGF). We currently have access to different biomarkers that can measure this risk in these patients, which are also considered to be possible direct mediators of the pathogenesis of atherosclerosis; such is the case for IL-8, IL-6, and tumour necrosis factor alpha (TNFα), which act as inflammatory markers, or interleukin 10, as anti-inflammatory marker.8,11 In a similar manner, altered levels of monocytes and cytokines derived from these molecules have been implicated in the inflammatory pathology associated with this disease. Several studies have suggested that elevated IL-8, IL-6, and TNFα levels are associated with greater morbidity and mortality rates. Decreases observed in serum cytokine profiles, which are produced after correcting 25OHD levels in these patients, support our hypothesis that correcting nutritional deficiencies of vitamin D could improve the inflammatory phenotype of terminal chronic kidney disease patients through non-traditional effects in circulating monocytes, and possibly in other tissues as well. This was reflected in the study by Stubbs et al.,15 in which 7 patients with deficient 25OHD levels on HD were treated with cholecalciferol, producing increased serum levels of this hormone, increased expression in monocytes of vitamin D receptors, decreased levels of 1-alpha-hydroxylase, and reduced circulating levels of inflammatory cytokines (IL-8, IL-6, and TNF). These results suggest that vitamin D therapy has a biological effect on excessive circulating inflammatory markers and monocytes in patients on renal replacement therapy. Our patients exhibited a decrease under both treatments in IL-8 values, a well-known inflammatory marker that is related to increased vascular calcification and morbidity/mortality rates. These results also appear to be associated with an improved response to ESA and improved resistance rates to EPO, probably in correlation with decreased inflammation.
In a systematic review and meta-analysis carried out by George et al.21 on the effect of vitamin D supplements on glycaemia, insulin resistance, progression of diabetes, and complications, the authors did not observe a significant improvement in fasting glucose levels, glycosylated haemoglobin, or insulin resistance in patients treated with vitamin D as compared to a placebo. These results are comparable to our own findings in terms of the benefits of treating both groups with 25OHD or paricalcitol and insulin resistance and HOMA index.22,23
Our study has some limitations. Firstly, our sample size was small (11 + 15 patients), and it may be that some of our results did not reach statistical significance would have with larger sample sizes. Our study sample was homogeneous in terms of treatment at a single centre and similar ages among patients, but there were differences with elevated standard deviations for some parameters. However, we would also point out the advantages of our study in that we were able to observe the isolated effects of each drug for three months followed by another three months using the same treatment scheme in all patients. This is also a novel study, in that no previous publication in the medical literature to our knowledge has examined both treatments in combination and their effects on bone-mineral metabolism, inflammation, and anaemia markers.
In light of our results, we can conclude that oral calcifediol supplements in patients on HD appear to be a safe measure to reduce vitamin D deficits. The provision of supplements for a selective activator of the vitamin D receptor, in this case paricalcitol, appears to achieve a greater control of SPTH as compared to calcifediol, alone reducing bone remodelling marker levels. We cannot conclude from our results that the combination of calcifediol and a selective activator of vitamin D receptors provides superior results to those obtained using each drug alone. It is evident that a prospective study with a larger sample size would be necessary to definitively address this question.
Table 1. Modification of the different study variables in both treatment groups over the course of the study
Figure 1. Variation in interleukin 8 levels over the course of the study in the paricalcitol group as compared to the group treated with calcifediol
Figure 2. Correlation between haemoglobin levels and resistance index to erythropoiesis-stimulating agents in the groups treated with paricalcitol and calcifediol over the course of the study