Background: Ghrelin is a novel 28 amino acid growth hormone-releasing peptide hormone that has been shown to inhibit cell proliferation and to decrease the production of proinflammatory cytokines by monocytes/macrophages. Moreover it decreases the release of endothelin-1 (ET-1), as well as mononuclear cell binding. Material and methods: Seventeen patients with proliferative glomerulopathies (PG) and 15 patients with non-proliferative glomerulopathies (NPG) were examined by percutaneous renal biopsy. As a control 11 biopsy specimens of the kidneys removed because of trauma were used. The immunoexpression of ghrelin and ET-1 was assessed semiquantitatively whereas the interstitial monocytes/macrophages and interstitial area were evaluated quantitatively. Results: The mean value of the immunoexpression of ghrelin was significantly diminished in PG patients as compared to both NPG group and controls while the mean values of ET-1, interstitial CD68+ cells, as well as interstitial area were in PG group increased in comparison with controls and NPG patients, most of them significantly. In all groups there were significant negative correlations between immunostaining of ghrelin and ET-1, whereas negative correlation between immunostaining of ghrelin and CD68+ cells was significant only in PG group. Conclusions: We can confirm the presence of ghrelin in tubular epithelial cells in normal and diseased human kidneys. Lack or low level of this protein in proliferative glomerulopathies may be, in part, responsible for interstitial accumulation of monocytes/macrophages in these cases.
Antecedentes: La grelina es un péptido de reciente descubrimiento de 28 aminoácidos que libera la hormona del crecimiento y que se ha demostrado que inhibe la proliferación celular al igual que disminuye la producción de citoquinas proinflamatorias mediante monocitos/macrófagos. Además, reduce la liberación de endotelina-1 (ET-1), así como la unión de células mononucleares. Materiales y métodos: Se practicó biopsia renal percutánea a diecisiete pacientes con glomerulopatías proliferatias (GP) y a quince pacientes con glomerulopatías no-proliferativas (GNP). Como grupo de control se utilizaron once biopsias de riñones que habían sido extirpados por traumatismo. La inmunoexpresión de la grelina y la ET-1 se determinó semicuantitativamente mientras que se realizaba un análisis cuantitativo de la zona intersticial y de los monocitos/macrófagos intersticiales. Resultados: El valor medio de la inmunoexpresión de la grelina se vio considerablemente disminuido en pacientes con GP, en comparación con el grupo de pacientes con GNP y de control, mientras que los valores medios de ET-1, células CD68+ intersticiales, así como de la zona intersticial, se vieron incrementados en el grupo de pacientes con GP en comparación con el grupo de control y los pacientes con GNP, la mayoría de ellos de forma significativa. En todos los grupos se observaron correlaciones negativas importantes entre la expresión de grelina y de ET-1, mientras que la correlación negativa entre la expresión de grelina y de células CD68+ era relevante únicamente en el grupo de pacientes con PG. Conclusiones: Puede confirmarse la presencia de grelina en las células epiteliales tubulares en riñones humanos normales y enfermos. La falta o el reducido nivel de esta proteína en las glomerolupatías proliferativas pueden ser, en parte, la causa de la acumulación intersticial de monocitos/macrófagos en estos casos.
INTRODUCTION
Ghrelin is a 28 amino acid peptide hormone, first discovered in stomach cell extracts, that promotes the release of growth hormone1,2. It is generated by processing of a 117 amino acid peptide, preproghrelin, by specific proteases and stored in secretory vesicles of endocrine cells3. Human ghrelin gene is located on chromosome 3 and consists of 4 exons and 3 introns4. A natural receptor for ghrelin (GHS-R) is a classical G-protein coupled receptor, consists of 336 amino acids and it was found to contain seven putative alpha-helical membrane spanning segments and three intracellular and extracellular loops5. Apart from the gastric mucosa ghrelin is also expressed in other tissues, such as hypothalamus, pituitary, pancreas, immune cells, lung, ovary, testes, intestine, placenta and various tumors3,5. Interestingly, Aydin et al.6 found that the urinary ghrelin level was higher than blood level, suggesting that the kidney might produce more ghrelin than stomach. It was also proposed that this locally produced ghrelin may modulate cell pathophisiology through an autocrine mechanism2. Ghrelin is a multifunctional molecule, involved in many biological processes3. Apart from the regulation of a growth hormone release it takes place in appetite regulation7 and gut motility8. Moreover, it was suggested that ghrelin inhibits cell proliferation9, decreases the production of proinflammatory cytokines by monocytes/macrophages10,11, endothelin-1 (ET-1) release12,13, and mononuclear cell binding11. In view of the above, the aim of the present study was to evaluate renal ghrelin immunoexpression in proliferative (PG) and so called non-proliferative glomerulopathies (NPG) as well as to find whether this immunoexpression could correlate with ET-1 immunoexpression, interstitial monocytes/macrophages and interstitial fibrosis.
MATERIAL AND METHODS
Patients
Kidney tissue biopsies were obtained for diagnostic purposes percutaneously from 17 patients in PG group (11 males and 6 females, aged 22-58, mean age = 39.7) and 15 from NPG patients (9 males and 6 females, aged 25-63, mean age = 46.9). PG group included 9 cases of IgA nephropathy with diffuse mesangial proliferation, 5 cases of mesangiocapillary glomerulonephritis type I and 3 cases of proliferative mesangial glomerulopathy with IgM depositions. In this group 6 patients showed hematuria alone, 4 nephrotic syndrome, 4 proteinuria <3.5 g/day and 3 poteinuria and hematuria. Renal function impairment (serum creatinine >1.5 mg/dl) was noted in 4 cases. NPG group incorporated 10 cases of membranous glomerulopathy and 5 cases of minimal change disease. In these patients nephrotic syndrome was seen in 8 cases, proteinuria <3.5 g/day in 4, proteinuria and hematuria in 3 and renal function impairment (serum creatinine >1.5 mg/dl) in 1. The mean duration of PG prior to biopsy taking was 6.3 months (range 4.7-8.2 months), and the mean duration of NPG prior to biopsy was 7.7 months (range 7.5-9 months). At the biopsy none of the patients had been treated with immunosuppressive drugs. As a control 11 biopsy specimens of the kidneys removed because of trauma were used (the male to female ratio was 7:4, the mean age was 38.1±7.2). None of the persons from whom renal tissue originated were known to have had previous or actual renal disease. Before the semiquantitative and quantitative examinations were carried out, all control specimens were histologically examined by a nephropathologist and found to be normal renal tissue.
In all cases, diagnosis of glomerulopathies were based on characteristic findings by light microscopy (sections stained with hematoxylin and eosin, Masson-Trichrome, Jones' silver impregnation and periodic acid-Schiff followed by Alcian Blue) as well as electron-microscopy and immunofluorescence using standard protocols. Thickness of each section was controlled according to the method described by Weibel14.
Immunohistochemistry
Paraffin sections were mounted onto superfrost slides, deparaffinized, then treated in a microwave oven in a solution of citrate buffer, pH 6.0 for 20 min at 750 W and transferred to distilled water. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in distilled water for 5 min, and then sections were rinsed with Tris-buffered saline (TBS, DakoCytomation, Denmark) and incubated with: rabbit-anti-human ghrelin antibody (Phoenix Pharmaceuticals. Inc., dilution 1:400), monoclonal mouse anti-human anti-endothelin-1 antibody (clone TR.ET.48.5, Sigma, Saint Louis, USA, dilution 1:250), and monoclonal mouse anti-human CD68 antibody (DakoCytomation, Denmark, dilution 1:100). Afterwards LSAB+/HRP Universal kit (DakoCytomation, Denmark) prepared according to the instructions of the manufacturer was used. Visualisation was performed by incubating the sections in a solution of 0.5 mg 3,3'-diaminobenzidine (DakoCytomation, Denmark), per ml Tris-HCl buffer, pH 7.6, containing 0.02% hydrogen peroxide, for 10 min. After washing, the sections were counter-stained with hematoxylin and coverslipped. For each antibody and for each sample a negative control were processed. Negative controls were carried out by incubation in the absence of the primary antibody and always yielded negative results. For ghrelin positive control was performed using gastric mucosa. In each specimen staining intensity of ghrelin in tubuli and ET-1 in the endothelium of peritubular capillaries as well as arterioles and in the renal tubular epithelial cells were recorded semiquantitatively by two independent observers in 7-10 adjacent high power fields and graded from 0 (staining not detectable), 1 (weak immunostaining), 2 (moderate immunostaining intensity) and 3 (strong staining). The mean grade was calculated by averaging grades assigned by the two authors and approximating the arithmetical mean to the nearest unity.
Morphometry
Histological morphometry was performed by means of image analysis system consisting of a PC computer equipped with a Pentagram graphical tablet, Indeo Fast card (frame grabber, true-color, real-time), produced by Indeo (Taiwan), and color TV camera Panasonic (Japan) coupled to a Carl Zeiss microscope (Germany). This system was programmed (MultiScan 8.08 software, produced by Computer Scanning Systems, Poland) to calculate the number of objects (semiautomatic function) and the surface area of a structure using stereological net (with regulated number of points).
The coloured microscopic images were saved serially in the memory of a computer, and then quantitative examinations had been carried out. Interstitial area was measured as a surface fraction in sections stained with Masson trichrome using point counting method which is an adaptation of the principles of Weibel14. The point spacing being 16 µm. Total numbers of the points of a net was 169, and total area was 36,864 sq. µm. Under the net described above 8-10 randomly selected adjacent fields of the renal cortex were investigated. Glomeruli and large blood vessels were neglected. The percentage interstitial area was an expression of the number of points overlying renal cortical interstitium as a percentage of the total points counted.
Interstitial monocytes/macrophages were determined by counting CD68+ cells (semiautomatic function) in a sequence of ten consecutive computer images of 400x high power fields - 0.0047 mm2 each. The only adjustments of field were made to avoid glomeruli and large vessels. The results were expressed as a mean number of CD68 immunopositive cells per mm2.
Statistical methods
All values were expressed as the mean ± SD (standard deviation). The differences between groups were tested using Student t-test for independent samples preceded by evaluation of normality and homogenity of variances with Levene's test. Additionally the Mann-Whitney U test was used where appropriate. Correlation coefficients were calculated using Spearman's method. Results were considered statistically significant if P <0.05.
RESULTS
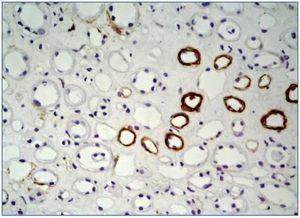
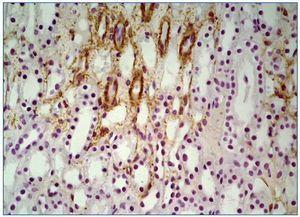
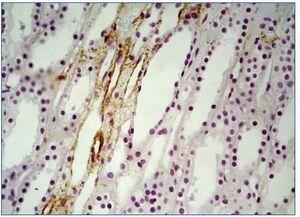
In all renal biopsy specimens in both controls and NPG groups ghrelin was detected in the renal tubular epithelial cells of thin portion of the Henle’s loops and some distal tubules. This immunoexpression was focal and confined to the cytoplasm (figure 1, figure 2). In 14 PG patients (3 cases were completely negative) only slight focal immunoexpression of ghrelin in tubular epithelial cells of thin portion of the Henle’s loops and distal tubules was seen (figure 3). In all groups ghrelin immunoexpression was absent from glomerular and interstitial areas.
The semiquantitative data of the immunoexpression of ghrelin in tubuli and ET-1 in the endothelium of peritubular capillaries and arterioles and in the renal tubular epithelium as well as morphometric data of the interstitial CD68+ cells and interstitial area appear from table 1. The mean value of the immunoexpression of ghrelin was significantly diminished in PG patients as compared to both NPG group and controls whereas this immunoexpression did not differ significantly between controls and NPG group. On the other hand the mean values of ET-1, interstitial CD68+ cells, as well as interstitial area were in PG group increased in comparison with controls and NPG patients, most of them significantly, meanwhile the differences between controls and NPG patients were not significant. The correlations between the tubular immunoexpression of ghrelin and ET-1, CD68+ cells as well as interstitial area are shown in table 2. In all groups there were significant negative correlations between immunostaining of ghrelin and ET-1, whereas negative correlation between immunostaining of ghrelin and CD68+ cells was significant only in PG group. The relationships between ghrelin and interstitial area were in all groups negative, but weak and not significant.
DISCUSSION
The reports on ghrelin in a kidney tissue are very scanty. In study of Gheraldoni et al. Ghrelin was not detectable in the kidney15. Mori et al.16 demonstrated the ghrelin immunoreactivity in the mouse kidney which was much more abundant then that in the mouse plasma. They concluded that ghrelin is produced locally in the kidney and suggested the possible endocrine and/or paracrine role of this peptide. Although these authors found that ghrelin gene was expressed in glomerulus and renal cells, in our study the glomeruli showed no ghrelin immunoexpression in all cases investigated, whereas epithelial tubular cells were focally positive. Similarly, Dagli et al.2 noticed ghrelin immunoexpression in tubular epithelium of human normal kidney, but glomeruli were absent from ghrelin immunoreactivity. In the kidneys of normal rats, mice, hamsters and diabetic rats positive ghrelin immunoexpression was observed in distal tubular epithelium17,18. There was no positive staining in the proximal tubules and glomeruli. However, to the best of our knowledge, this is the first study of ghrelin immunoexpression in human glomerulopathies.
We found immunoexpression of ghrelin in normal kidneys and in both proliferative glomerulopathies usually accompanied with prominent interstitial leukocyte infiltrates and non proliferative with only a little interstitial damage. However, the major finding in the present study was the observation that tubular immunoreactivity of ghrelin in proliverative glomerulopathies was significantly diminished as compared to non proliferative patients and controls. Emerging evidence indicates that ghrelin has anti-inflammatory activity19-21 as it decreases the release of proinflammatory cytokines from T cells and monocytes22. It was documented that ghrelin administration in mice significantly decreased serum TNF-alpha, IL-1 beta and IL-6 and as well as had protective effect against endoxemia-induced kidney injury13. This raises the interesting possibility that low level or lack of ghrelin in proliferative glomerulopathies might have a role in interstitial damage in these cases which in part may also depend on ET-1. We found that immunoexpression of ET-1 was significantly increased in PG as compared to controls. In comparison to NPG, however, this difference was not significant. Moreover, in the present study there were negative significant correlations between immunoexpression of ghrelin and ET-1 in all groups investigated. Endothelin is a potent biological mediator which exists in three isoforms: endothelin-1, -2 and -3, however ET-1 is considered the clinically most important endothelin in human kidney disease13,24. The main vascular effect of ET-1 is transient vasodilatation and profound and sustained vasoconstriction25. It is also regarded as a potent mitogen for mesangial cells and as a pro-fibrotic protein26,27. On the other hand ghrelin is known to inhibit ET-1 release probably via TNF-alpha pathway12,13, thus high immunoexpression of ET-1 in PG patients may suggest, among others, an involvement of this protein in interstitial renal damage in these cases.
As might be expected interstitial monocytes/macrophages were in PG group significantly more numerous than in NPG and controls. Moreover, CD68+ cells in PG patients significantly, negatively correlated with the immunoexpression of ghrelin. Monocytes/macrophages seem to be the major cell targets in the inhibition of the high mobility box 1 secretion in which ghrelin blocked its cytoplasmic translocation21. In vitro study of Li et al.11 showed that ghrelin inhibited mononuclear cell binding, whereas findings of Chen et al.10 documented that ghrelin attenuates proinflammatory cytokine production in lung macrophages in rats. Our present finding suggests the interesting opportunity that ghrelin might have the potential to reduce monocytes/macrophages recruitment in the kidney tissue.
Renal interstitial fibrosis is the final common pathway leading to end-stage disease in various nephropathies. We found the interstitial area to be significantly increased in PG as compared to NPG and controls. Although the correlations between interstitial area and ghrelin were in all groups negative, they were weak and not significant, thus no casual associations can be made between these parameters.
In conclusion, we can confirm the presence of ghrelin in tubular epithelial cells in normal and diseased human kidneys. Lack or low level of this protein in proliferative glomerulopathies may be, in part, responsible for interstitial accumulation of monocytes/macrophages in these cases.
Acknowledgement
This work was supported by grant No NN402088735
Table 2. The correlations between renal immunoexpression of ghrelin and selected parameters in PG, NPG and controls
Figure 1. Strong focal immunoexpression of ghrelin in tubular epithelium of thin portion of the Henles loops in control case. x200.
Figure 2. Intense focal immunoexpression of ghrelin in epithelial cells of thin portion of the Henles loops and several distal tubuli in NPG case. x200.
Figure 3. Weak, focal immunoexpression of ghrelin in tubular epithelium of thin portion of the Henles loops and distal tubuli in PG patient. x200.
Table 1. Renal immunoexpression of ghrelin, ET-1, and analysis of interstitial volume as well as CD68+ cells in PG, NPG and controls