The impact of acute rejection in kidney graft survival is well known, but the prognosis of other diagnoses is uncertain. We evaluated the frequency and impact on graft survival of different diagnostic categories according to the Banff 2013 classification in a cohort of renal transplant recipients.
Material and methodsRetrospective study of 495 renal biopsies by indication in 322 patients from 1990 to 2014. Two independent observers reviewed the histological reports, reclassifying according to the Banff 2013 classification.
ResultsOf 495 biopsies, 28 (5.7%) were not diagnostic. Of the remaining 467, 10.3% were “normal” (category 1), 19.6% antibody-mediated changes (category 2), 5.9% “borderline” changes (category 3), 8.7% T-cell-mediated rejection (category 4), 23.4% interstitial fibrosis/tubular atrophy (IFTA) (category 5) and 26.5% with other diagnoses (category 6). As time after transplantation increases, diagnoses of categories 1, 3 and 4 decrease, while categories 5 and 2 increase. Worse graft survival with category 2 diagnosis was observed (45% at 7.5 years, HR 4.29 graft loss [95% CI, 2.39–7.73]; P≤0.001, compared to category 1). Grafts with “unfavourable histology” (chronic antibody-mediated rejection, moderate-severe IFTA) presented worse survival that grafts with “favourable histology” (normal, acute tubular necrosis, mild IFTA).
ConclusionsThe Banff 2013 classification facilitates a histological diagnosis in 95% of indication biopsies. While diagnostic category 6 is the most common, a change in the predominant histopathology was observed according to time elapsed since transplantation. Antibody-mediated changes are associated with worse graft survival.
El impacto del rechazo agudo en la supervivencia del injerto renal es bien conocido; sin embargo, el pronóstico de otras entidades es incierto. Evaluamos la frecuencia y el impacto en la supervivencia del injerto de las diferentes categorías diagnósticas según la clasificación Banff 2013 en una cohorte de trasplantados renales y su impacto en la supervivencia del injerto.
Material y métodosEstudio retrospectivo de 495 biopsias renales por indicación, en 322 pacientes entre 1990 y 2014. Dos observadores independientes revisaron los diagnósticos histológicos y reclasificaron según Banff 2013.
ResultadosDe 495 biopsias, 28 (5,7%) fueron no diagnósticas. De las 467 restantes, 10,3% fueron «normales» (categoría 1), 19,6% fueron cambios mediados por anticuerpos (categoría 2), 5,9% fueron cambios borderline (categoría 3), el 8,7% fueron rechazo mediado por células T (categoría 4), el 23,4% fue fibrosis intersticial/atrofia tubular (FIAT) (categoría 5) y el 26,5% fueron otros diagnósticos (categoría 6). Al aumentar el tiempo postrasplante, disminuyen los diagnósticos de categorías 1, 3 y 4 y aumentan los de la 5 y la 2. Observamos peor supervivencia en injertos con diagnósticos de categoría 2 (45% a 7,5 años; HR pérdida del injerto 4,29 [IC 95%: 2,39-7,73]; p≤0,001, con respecto a categoría 1). Los injertos con «histología desfavorable» (rechazo crónico mediado por anticuerpos, IFTA moderada-severa) presentan peor supervivencia que los injertos con «histología favourable» (normal, necrosis tubular aguda, FIAT leve).
ConclusionesLa clasificación de Banff 2013 permite el diagnóstico histológico en el 95% de las biopsias por indicación. La categoría 6 es la más frecuente, pero se observa una modificación en la histopatología predominante según el tiempo postrasplante. Los cambios mediados por anticuerpos se asocian con peor supervivencia del injerto.
Due to the development of new immunosuppressive therapies in recent decades, the incidence of acute rejection has decreased considerably.1 Short-term graft survival has also improved, going from a 9.1% probability of all-cause graft loss in the first year post-transplant in 2000 to 7.7% in 2011.1 However, this benefit is not reflected in a significant improvement in long-term graft survival.2–4
It is necessary to seek strategies to improve long-term kidney graft survival. It is essential to study the causes and mechanisms involved in graft loss and to identify prognostic markers of graft loss better than those used classically, such as the estimated glomerular filtration rate or proteinurial. Although some studies have correlated a low estimated glomerular filtration rate after one year of transplantion with a higher rate of long-term graft failure, its predictive value for graft loss is limited.5–7 Proteinuria is also a kidney function marker with actual and predictive value; but the values of proteinuria may be affected by many factors such as infections, intercurrent diseases, etc., and its onset often reflects previously established kidney damage.8
Renal biopsy (RB) provides very valuable information in terms of diagnostic and prognostic assessment, as reflected in previous studies mostly based on RB performed by protocol, which allows the identification of different lesions of histological patterns that are associated with worse graft survival.9–14 Thus, the Banff classification and the updates are based on the advances in the understanding of the pathophysiological mechanisms involved in renal graft injury. Lesions that previously had no diagnostic or prognosis value can be catalogued following a universal language, becoming a fundamental tool for assessing renal graft biopsies.15–23
However, there is little data from studies based on biopsies performed with indication, which focused primarily on establishing a relationship between a diagnosis of T cell-mediated rejection24,25 or antibody-mediated rejection with a worse graft outcome,26–28 and no information, about the prognostic value for the rest of the histological patterns.
The objective of this study was to analyse the RBs with indication conducted in a cohort of kidney transplant patients. Then, reclassify these RB according to the recent Banff 2013 classification,23 and to establish the prognostic value of graft survival according to the different diagnostic categories.
MethodsA retrospective study was conducted based on the findings from 495 transplant RBs, performed with indication (kidney function deterioration, proteinuria, or microhaematuria), in 322 patients at the Hospital del Mar (Barcelona, Spain) between 1990 and 2014.
The histopathological reports from those biopsies were reviewed by two authors of the present manuscript (DRP and MJPS) who reclassified the histological findings according to the Banff 2013 classification categories23: category 1: normal RB, category 2: antibody-mediated changes, category 3: borderline changes, category 4: T cell-mediated changes, category 5: interstitial fibrosis and tubular atrophy (IFTA), and category 6: other (changes not considered secondary to rejection). In such a case that histopathological reports contained insufficient information to be reclassified into any of the above described categories (n=37), two pathologists (JG and ISG) reassessed the available tissue samples and performed additional histological techniques required to made specific diagnosis (mostly C4d staining) If the reports contained data compatible with several categories, the predominant histological findings with the greatest prognostic weight were taken into account for the purpose of assigning a single category to each biopsy; therefore the diagnoses were exclusive.
Furthermore, aiming of categorise the diagnoses with a potential association with a better or worse renal prognosis, the histological diagnoses were divided into two groups: 1) favourable histology: which included samples with normal histology or with minimal changes, acute tubular necrosis (ATN), or mild IFTA; 2) unfavourable histology: which included cases with diagnoses of chronic antibody-mediated rejection (samples with transplant glomerulopathy (cg>0)±positive C4d staining (focal or diffuse) in peritubular capillaries ± peritubular capillary basement membrane multi-layering ± new onset intimal fibrosis with no other attributable cause) or moderate-severe IFTA. This division was made based on previous studies relating these histological patterns with worse graft survival.14,29
Patients were followed from the time of the RB until loss of the graft (defined as return to dialysis or retransplant) or the patient's death. The statistical study included a Kaplan–Meier survival curve, using the log-rank test. Cox regression was used to calculate the risk of graft loss. The level of significance was set at p<0.05 and 95% for the confidence interval. The SPSS software package version 20.0 was used for the statistical calculations.
ResultsThe analysis included 495 RBs from 322 patients, 62.7% of them male, with a mean age at the time of the kidney transplant (KT) of 47.2±13.8 years. The median time from KT until the RB was 12 months [IQR 1–51.5]. The median follow-up from the time of the RB was 21 months [IQR 7–65]. Histological diagnoses were reclassified according to the Banff 2013 classification categories. The histological findings did not allow for a conclusive diagnosis in 28 RBs (5.7%). Of the remaining 467, 51 (10.9%) were classified as normal (category 1), 97 (20.8%) as changes mediated by antibodies (category 2), 29 (6.2%) as borderline changes (category 3), 43 (9.2%) as T cell-mediated rejection (category 4), 116 (24.8%) as IFTA (category 5), and 131 (28.1%) as other diagnoses (category 6) (Table 1).
Overall results of the kidney transplant recipients to those who underwent renal biopsy with indication.
| Number of renal biopsies | 495 (322 patients) |
|---|---|
| Banff 2013 diagnosis (n, %) | 467 (94.3) |
| Male (n, %) | 300 (60.6) |
| Age in years (mean, standard deviation) | 47.2±13.8 |
| KT-RB time in months (median, IQR) | 12 [1–51.5] |
| Post-RB follow-up in months (median, IQR) | 21 [7–65] |
KT: kidney transplant; IQR: interquartile range; RB: renal biopsy.
Among the category 5 (IFTA) biopsies, 63 (54.4%) were graded as mild, 30 (25.8%) moderate, and 23 (19.8%) severe. Among the category 6 or other diagnoses, the most common histological diagnoses were ATN in 53 cases (40.5%), followed by recurrent glomerular disease in 24 (18.3%) (Table 2).
Overall distribution of biopsies according to the Banff 2013 categories.
| Banff 2013 category | N (%) |
|---|---|
| Normal (category 1) | 51 (10.9) |
| Antibody-mediated changes (category 2) | 97 (20.8) |
| Borderline changes (category 3) | 29 (6.2) |
| T cell-mediated rejection (category 4) | 43 (9.2) |
| IFTA (category 5) | 116 (24.8) |
| Mild | 63 (54.4) |
| Moderate | 30 (25.8) |
| Severe | 23 (19.8) |
| Other diagnoses (category 6) | 131 (28.1) |
| ATN | 53 (40.5) |
| Kidney disease relapse | 24 (18.3) |
IFTA: interstitial fibrosis/tubular atrophy; ATN: acute tubular necrosis.
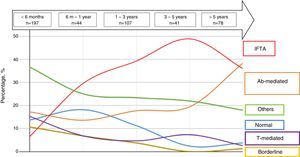
The most common diagnoses were analysed relative to time after transplant. During the first years after the transplant a large proportion of biopsies were classified within categories 1, 3, 4, and 6; with more prolonged time after transplantation the high proportion of categories 1, 3, 4, and 6 was reduced in favour of an increase in biopsies classified as categories 2 and 5 (Fig. 1, Table 3).
Distribution of renal biopsies according to time after kidney transplant.
| Diagnostic categories | <6 months n=197 (42.2%) | 6–12 months n=44 (9.4%) | 1–3 years n=107 (22.9%) | 3–5 years n=41 (8.7%) | >5 years n=78 (16.7%) |
|---|---|---|---|---|---|
| Normal biopsy | 27 (13.7) | 8 (18.2) | 12 (11.2) | 1 (2.4) | 3 (3.8) |
| Antibody-mediated changes | 34 (17.2) | 6 (13.6) | 19 (17.7) | 8 (19.5) | 30 (38.4) |
| Borderline changes | 21 (10.6) | 3 (6.8) | 4 (3.7) | 0 | 1 (1.2) |
| T cell-mediated rejection | 30 (15.2) | 3 (6.8) | 5 (4.6) | 3 (7.3) | 2 (2.5) |
| IFTA | 13 (6.6) | 13 (29.5) | 42 (39.2) | (48.8) | (35.8) |
| Others | 72 (36.5) | 11 (25.0) | 36 (23.3) | 9 (21.9) | 14 (17.9) |
IFTA: interstitial fibrosis/tubular atrophy.
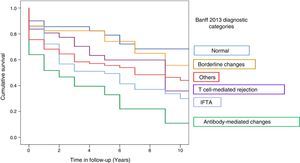
Graft survival (censored by death) 21 months after the RB, according to the Banff 2013 category in which RB was classified was: 70% for category 1, 45% for category 2, 69% for category 3, 56% for category 4, 51% for category 5, and 50% for category 6 (Fig. 2).
The risk of graft loss according to the category (taking category 1 as the reference) was highest in category 2 (HR 4.29, 95% CI: 2.39–7.73; p<0.001) and category 5 (HR 2.4; 95% CI: 1.36–4.27; p=0.003). The impact of T cell-mediated rejection on graft survival was not significant (HR 0.98; 95% CI: 0.90–3.19; p=0.98) (Table 4).
Risk of graft loss as compared to Banff 2013 category 1 (normal biopsy or with minimal changes).
| Categories | HR | CI (95%) | p |
|---|---|---|---|
| 1: Normal | 1 | ||
| 2: Antibody-mediated changes | 4.29 | 2.39–7.73 | <0.001 |
| 3: Borderline changes | 1.22 | 0.53–2.79 | 0.638 |
| 4: T cell-mediated rejection | 0.98 | 0.90–3.49 | 0.98 |
| 5: IFTA | 2.41 | 1.36–4.27 | 0.003 |
| 6: Others | 2.04 | 1.16–3.59 | 0.013 |
IFTA: interstitial fibrosis/tubular atrophy.
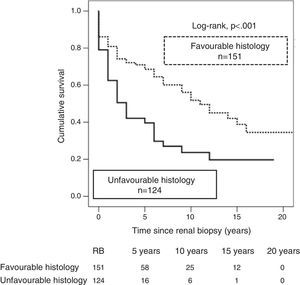
According to the previously described as histological diagnoses with high prognostic value,26 276 RBs were classified as having a favourable (n=152) or unfavourable histology (n=124). A better graft survival was observed with biopsies in the favourable histology category as compared with those with unfavourable histology (35 vs. 20%; p<0.001), and the risk of graft loss was twofold with unfavourable histology (HR 2.2; 95% CI: 1.51–3.14; p<0.001) (Fig. 3).
DiscussionIn this study we have analysed the results from a wide series of RBs with indication. We reanalysed them according to the Banff 2013 classifications and established what was the prognosis in terms of renal survival in each diagnostic category. The findings compatible with antibody-mediated rejection or moderate-severe IFTA confer the highest risk of graft loss.
The objective of the first meeting of pathologists and nephrologists held in Banff in 1991 was to define and classify the histopathological findings found in kidney transplant biopsies. Until that time, the only diagnosis that was significant and treatable was acute rejection. In subsequent meetings, the diagnostic categories were updated, adapting them to advances in the understanding of the pathophysiology of graft loss and to a better characterisation of antibody-mediated injury. A correct classification of the histopathological diagnoses may clarify clinical conditions previously not well-defined or even underdiagnosed. In this study, the RB were reclassified according to Banff 201323 and it made possible to obtain a specific histological diagnosis in 95% of the biopsies, which illustrate the high diagnostic efficiency obtained with the RBs by performed with indication. The most common category was “other diagnoses” (28%), and within that category, diagnoses of ATN and recurring glomerular disease were most frequent. The next most common category was IFTA (24.8%), categorised as mild in 50% of our cases. Prior studies conducted in biopsies with indication showed heterogeneous results regarding the most common diagnoses. In a multi-centre study, Sellares et al.26 prospectively assessed a cohort of 315 KT recipients in whom a RB had been performed due to graft dysfunction (412 biopsies) to identify the losses and to try to assign a cause to each of them. They reported that the most common category was a normal RB, or one with no big abnormalities in 29%, following by antibody-mediated changes in 18%.
Sis et al.30 evaluated 234 biopsies from 173 KT patients performed 16 months (as a mean) after the transplant; using the Banff 2007 classification criteria, they found that the most common diagnosis was T cell-mediated rejection (19%), followed by “other diagnoses”(17%). In this study, IFTA and C4d-positive antibody-mediated rejection accounted for only 5% and 7% of the diagnoses, respectively.
Therefore, based on our data, the overall analysis of the frequency of the different diagnoses would not provide valuable prognostic information, since, as we can observe in both this study and others, the most common diagnoses varied substantially depending on the time elapsed after transplant. In our cohort, we observed that categories such as “normal” biopsy, ATN, and T cell-mediated changes were detected at a higher frequency in the RBs performed during the first year after the transplant. T cell-mediated changes were found in 15% of the biopsies performed during the first 6 months, whereas in biopsies performed 5 years after the transplant, this diagnosis only accounted for 2.5%. Furthermore, diagnoses such as antibody-mediated damage and chronic damage from different causes31 (represented by IFTA) were more commonly found in late biopsies. In the case of antibody-mediated damage, it increased from 17.2% of RBs performed before the 6th month to 46% of biopsies performed between the 3rd and 5th year after transplant. In the case of IFTA, it also increased from 6.6% in early biopsies to 36% in biopsies after 5 years. Sellares et al. reported similar results, with a clear increase in the frequency of a histological diagnosis of antibody-mediated rejection in relation to the time passed after transplant and a decrease in the frequency of T cell-mediated rejection.26
By analysing the prognosis of graft loss according to the assigned Banff 13 diagnostic category, we observe that as previously described, the risk of graft loss is fourfold increased in the transplants with RBs compatible with antibody-mediated rejection.14,26–28,32 The other histological pattern that was correlated with worse survival was moderate-severe IFTA, which does not correspond to a specific diagnosis but rather to a histological description with no weight of specificity; therefore, for a correct characterisation of the patient with IFTA it is necessary to obtain better diagnostic and prognostic information. On these lines, El-Zhogbi et al. found that 30.7% of kidney biopsies with graft loss had an IFTA diagnosis.32 When reassessing the RBs, 80.9% of these generic IFTA diagnoses could be attributed to a specific cause, such as BK polyomavirus-induced nephropathy, antibody-mediated rejection, or multiple episodes of acute cellular rejection.
Despite the fact that classically, acute rejection has been correlated with worse graft survival,24,25 in our kidney transplant cohort T cell-mediated rejection and a diagnosis of borderline changes had no significant impact on the kidney graft prognosis, which indicates that the weight classically attributed to this condition regarding its impact on graft survival could be categorized into antibody-mediated rejection. Excluding acute cellular rejection and antibody-mediated rejection from the survival analysis, the group including chronic antibody-mediated rejection and the presence of moderate-severe IFTA (unfavourable histology) shows a double risk of graft loss than the favourable histology. Therefore, these two conditions are postulated to provide the highest specific weight when predicting long-term graft survival.
The main limitation of our study is the retrospective nature of the biopsy analysis; this made difficult to obtain complete information to determine whether the findings obtained in the RBs were the direct cause of the graft loss. Therefore, a causal relationship could not be establish.
However, RB with indication continues to be the basic diagnostic tool for studying graft dysfunction. We did not find any published studies that analyse the prognostic value according to the different Banff 2013 categories. Our study provides important information about the specific weight on graft survival of these categories in a wide cohort of kidney transplant patients with a suitable follow-up time.
In summary, the Banff 2013 classification enables specific diagnoses to be reassigned to previously unclassifiable biopsies. In our study, this established a higher percentage in the category “other diagnoses”, followed by the IFTA category and the antibody-mediated damage category. These last two categories emerged as the diagnoses with the greatest negative impact on graft survival. Moreover, we detected a very significant number of biopsies with advanced chronic damage, represented in the form of IFTA, without being able to attribute an aetiology. This likely illustrate the deficiencies of using histology only when analysing the causes of graft damage, and the limitation of biopsies with indication obtained for early detection of phenomena leading to chronic damage and reduction of graft survival. The strategies used for studying kidney damage and graft surveillance should combine clinical, analytical, and histological variables along with new techniques to achieve an early detection of specific causes responsible for graft damage.
Authors/contributorsAll three authors contributed equally in the development of the study and in drafting the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Arias-Cabrales C, Redondo-Pachón D, Pérez-Sáez MJ, Gimeno J, Sánchez-Güerri I, Bermejo S, et al. Supervivencia del injerto renal según la categoría de Banff 2013 en biopsia por indicación. Nefrología. 2016;36:660–666.