The cause of vitamin D deficiency in chronic kidney disease (CKD) is probably multi-factorial; however, the relative importance of each potential determinant is uncertain.
AimsTo determine factors associated with serum levels of 25-hydroxy vitamin D (25OHD) and their relative importance in a cohort of pre-dialysis CKD patients.
Materials and methodsIncident patients admitted to a CKD outpatient clinic were included. Those who were receiving vitamin D supplements or anticonvulsants were excluded. In addition to demographic and clinical data, information about outdoor physical activity, season of blood collection, prescription of statins, anti-angiotensin drugs and xanthine-oxidase inhibitors were included as potential determinants. Johnson's relative weights analysis was used to estimate the relative importance of each potential determinant and the results were expressed as percentage contribution to multiple R.
ResultsThe study group consisted of 397 patients, 30 of whom were excluded. The mean serum level of 25OHD was 13.7±7.4ng/ml, and 81% of patients had serum levels lower than 20ng/ml. By multiple linear regression and relative weights analyses, the best determinants of low serum 25OHD levels and their relative importance were: higher proteinuria (28.5%), old age (21.4%), low physical activity (19.4%), female gender (19.3%) and low serum bicarbonate levels (11.4%).
ConclusionsProteinuria and age are the determinants with the highest relative importance for predicting 25OHD levels in CKD patients.
El origen de la carencia de vitamina D en la enfermedad renal crónica (ERC) parece multifactorial, pero es incierta la importancia relativa de cada uno de sus potenciales determinantes.
ObjetivosDeterminar los factores asociados a los niveles de 25-hidroxi-colecalciferol (25OHD) y su importancia relativa en una cohorte de pacientes con ERC prediálisis.
Material y métodosSe incluyeron pacientes incidentes en una consulta de ERC, excluyendo a aquellos que recibían suplementos de vitamina D o anticonvulsivantes. Además de los datos demográficos y clínicos, se analizó la influencia de la actividad física, estación del año de la extracción, y tratamiento con estatinas, antiangiotensina e inhibidores de la xantino-oxidasa. Para la estimación de la importancia relativa se utilizó el método de ponderación relativa de Johnson, expresando los resultados como porcentajes de contribución al R múltiple.
ResultadosSe estudiaron 397 pacientes, de los cuales 30 fueron excluidos. La concentración media de 25OHD fue de 13,7±7,4ng/ml, presentando unos niveles inferiores a 20ng/ml el 81% de los pacientes. Por regresión lineal múltiple y ponderación relativa, los principales determinantes de unos niveles más bajos de 25OHD fueron por orden de importancia: una mayor proteinuria (28,5%), mayor edad (21,4%), disminución de la actividad física (19,4%), sexo femenino (19,3%), y menor bicarbonato sérico (11,4%).
ConclusiónLa magnitud de la proteinuria y la edad son los factores con mayor importancia relativa como determinantes de los niveles de 25OHD en la ERC.
In recent years, interest in diagnosing vitamin D deficiency by measuring 25-hydroxycholecalciferol (25(OH)D) in serum has increased significantly. A significant proportion of the general population has low levels of 25(OH)D,1,2 but deficiency is found in most patients with chronic conditions, especially chronic kidney disease (CKD).3–8
The cause of the deficiency would seem to be multifactorial. Factors related to exposure to ultraviolet light (UV),7,9,10 and comorbidity such as diabetes11–13 may explain much of the deficit of vitamin D. In patients with CKD, the reduction in glomerular filtration rate,14–16 proteinuria3,17–19 and commonly used drugs20 may also have a significant influence. However, the relative individual importance of all these factors in vitamin D deficiency is unknown.
Multiple linear regression analysis is the method of choice to establish the degree of variation of a dependent variable using the information offered by independent variables.
One of the assumptions that must be met for prediction models to be reliable and accurate is that independent variables have no significant correlation between each other.21 This requirement is very difficult to meet when analysing the potential determinants of vitamin D levels in a population with CKD. There is a high degree of association between factors such as physical activity or exposure to ultraviolet radiation (UV) and age, gender and comorbidity, or between proteinuria and the prescription of certain drugs such as angiotensin inhibitors and receptor blockers. This complicates the interpretation and significance of the regression coefficients and the relative importance of each of these potential determinants.
One way of overcoming the difficulties generated by the multicollinearity in a multivariate linear regression model is determination, in addition to the standardized coefficients (beta), of the structure coefficients which provide additional information about the bivariate relationship between a predictor and the observed effect, without the influence of other predictors in the model.21–23
“Weighting of the relative importance” is an accredited method for establishing the proportional contribution of each predictor to the coefficient of determination (R2), correcting the effects of the cross-correlation between predictors.21–23
The aim of this study was to analyze the determinants of 25(OH)D levels in a cohort of patients with advanced CKD not on dialysis, and establish the relative importance of the variables found to be part of the best predictive model.
Materials and methodsPatientsAll incident patients in an advanced CKD clinic between February 2012 and March 2015 were included consecutively. All were over 18 and resided in the province of Badajoz, Spain. Patients being treated with vitamin D supplements (ergocalciferol, cholecalciferol or 25-hydroxycholecalciferol), or with drugs that interact with 25(OH)D metabolism, such as carbamazepine, were excluded from the study.
The following data were gathered when taking the medical history from the patient or from their medical notes and included as potential covariates in the analysis: age; gender; body mass index; Davies comorbidity index; date of extraction; reduction in physical activity; vitamin D analogs; statins; angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin-receptor antagonists (ARA); and xanthine oxidase (XO) inhibitors (allopurinol or febuxostat).
According to the time of year of the extraction, we put together a qualitative ordinal variable in which winter was scored as 0, autumn 1, spring 2 and summer 3.
Decrease in physical activity was considered when the patients reported no physical activity outside their homes and little exposure to the sun.
Laboratory testsThe 25(OH)D concentrations were measured using the electrochemiluminescence method (LIAISON® 25 OH Vitamin D TOTAL Assay, DiaSorin Inc., Stillwater, USA). The measuring range of this method covers concentrations from 4 to 150ng/ml, with intra-assay imprecision (coefficient of variation) of ≤5.5% and functional sensitivity of 4ng/ml.
Other biochemical parameters analyzed as potential determinants of 25(OH)D levels were: glomerular filtration rate (MDRD); serum albumin (immunoturbidimetric, ADVIA 2400 Chemistry System, Siemens Healthcare Diagnostics, USA); high-sensitivity C-reactive protein (turbidity meter, Siemens Healthcare Diagnostics); and proteinuria measured in 24h urine collection, expressing the results as g of protein per g of creatinine excretion. Also included as variables were serum uric acid concentrations (ADVIA, Siemens) and venous bicarbonate (ABL800 FLEX gas analyser, Radiometer Ibérica).
Serum calcium, phosphorus, magnesium and PTH concentrations were not considered as potential determinants of 25(OH)D levels.
Statistical methodsTo establish the best model of association between 25(OH)D levels (dependent variable) and the independent variables, multivariate linear regression was used. Independent variables were selected by an automatic stepwise selection procedure. In addition to the coefficient of multiple determination (R2) of the model, standardized partial regression coefficients (beta) were also calculated.
The best resulting model was assessed for multicollinearity, this being considered significant if the condition number was greater than 20, according to the Belsley et al. criteria.24
To determine the relative importance of each independent variable in the model, we calculated structure coefficients and performed a Johnson relative weights analysis and significance tests in order to compare the relative importance of the predictors.22,23 The relative importance of each variable is expressed as a percentage of contribution to multiple R.
For comparisons of continuous variables, Student's t test, ANOVA or Mann-Whitney test were used, according to the distribution characteristics of the variable. We used the χ2 test for comparison of categorical variables.
The results are expressed as mean or median and standard deviation or ranges respectively. p<0.05 was considered to be statistically significant.
Statistical analyses were performed with the IBM-SPSS Statistics 21.0 program (IBM Corp. Armonk, USA). For the Johnson relative weights analysis, we used the FIRE program created by Lorenzo-Seva and Ferrando.23 This program uses an SPSS Syntax Editor window, through which the calculations are performed.
ResultsThe total number of patients studied was 397, but 30 were excluded because they were already receiving vitamin D supplements (27 patients) or were being treated with carbamazepine (3 patients).
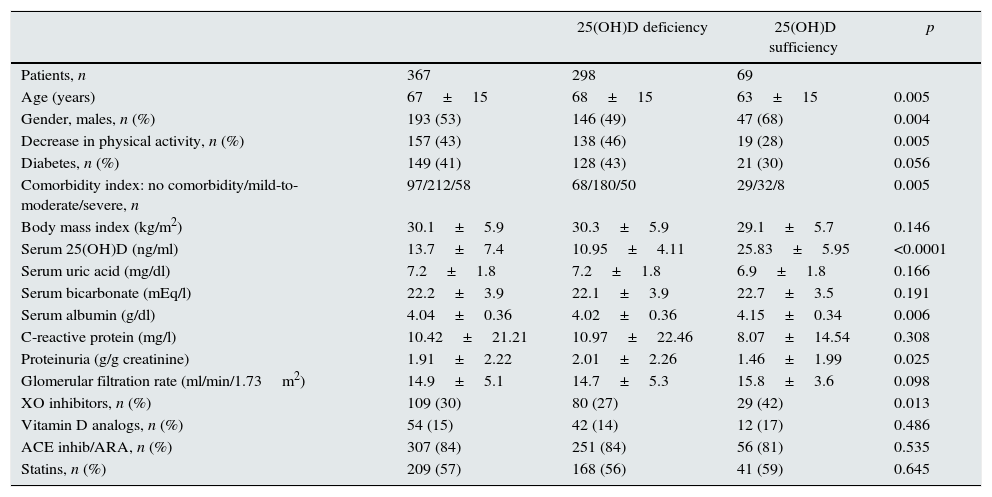
The characteristics of the remaining 367 patients who made up the study group are shown in Table 1. The total number of patients with serum 25(OH)D levels below 20ng/ml was 298 (81%).
Characteristics of the whole study group and subgroups according to whether they had serum levels of 25-hydroxycholecalciferol (25(OH)D) below (deficiency) or above (sufficiency) 20ng/ml.
| 25(OH)D deficiency | 25(OH)D sufficiency | p | ||
|---|---|---|---|---|
| Patients, n | 367 | 298 | 69 | |
| Age (years) | 67±15 | 68±15 | 63±15 | 0.005 |
| Gender, males, n (%) | 193 (53) | 146 (49) | 47 (68) | 0.004 |
| Decrease in physical activity, n (%) | 157 (43) | 138 (46) | 19 (28) | 0.005 |
| Diabetes, n (%) | 149 (41) | 128 (43) | 21 (30) | 0.056 |
| Comorbidity index: no comorbidity/mild-to-moderate/severe, n | 97/212/58 | 68/180/50 | 29/32/8 | 0.005 |
| Body mass index (kg/m2) | 30.1±5.9 | 30.3±5.9 | 29.1±5.7 | 0.146 |
| Serum 25(OH)D (ng/ml) | 13.7±7.4 | 10.95±4.11 | 25.83±5.95 | <0.0001 |
| Serum uric acid (mg/dl) | 7.2±1.8 | 7.2±1.8 | 6.9±1.8 | 0.166 |
| Serum bicarbonate (mEq/l) | 22.2±3.9 | 22.1±3.9 | 22.7±3.5 | 0.191 |
| Serum albumin (g/dl) | 4.04±0.36 | 4.02±0.36 | 4.15±0.34 | 0.006 |
| C-reactive protein (mg/l) | 10.42±21.21 | 10.97±22.46 | 8.07±14.54 | 0.308 |
| Proteinuria (g/g creatinine) | 1.91±2.22 | 2.01±2.26 | 1.46±1.99 | 0.025 |
| Glomerular filtration rate (ml/min/1.73m2) | 14.9±5.1 | 14.7±5.3 | 15.8±3.6 | 0.098 |
| XO inhibitors, n (%) | 109 (30) | 80 (27) | 29 (42) | 0.013 |
| Vitamin D analogs, n (%) | 54 (15) | 42 (14) | 12 (17) | 0.486 |
| ACE inhib/ARA, n (%) | 307 (84) | 251 (84) | 56 (81) | 0.535 |
| Statins, n (%) | 209 (57) | 168 (56) | 41 (59) | 0.645 |
No significant differences were found in the 25(OH)D concentrations according to the time of year the extraction was made (winter 13.8±8.1ng/ml; autumn 12.9±6.5ng/ml; spring 13.7±7.2ng/ml; and summer 14.1±6.7ng/ml; p=0.886, ANOVA).
Table 1 also shows the differences between patients with sufficiency or lack of vitamin D. The most notable characteristics of those with vitamin D deficiency were as follows: older women; more comorbidity; less physical activity; a more pronounced reduction in average serum albumin concentration and increased proteinuria, with no significant differences in the estimated glomerular filtration rate; and, to a lesser extent, being treated with xanthine oxidase inhibitors.
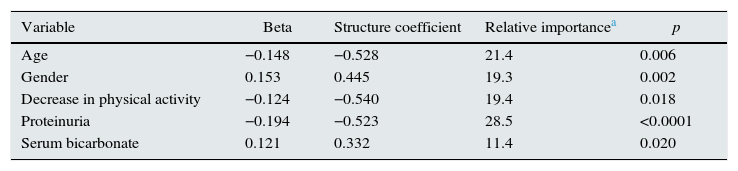
By multivariate linear regression, the best predictive model for 25(OH)D levels consisted of age, gender, decreased physical activity, proteinuria and serum bicarbonate (Table 2). Variables that were not part of the best equation but came close in their significance were the comorbidity index, serum uric acid and treatment with xanthine oxidase inhibitors (p=0.05).
Multiple linear regression and relative weighting. Factors best associated with 25(OH)D levels.
| Variable | Beta | Structure coefficient | Relative importancea | p |
|---|---|---|---|---|
| Age | −0.148 | −0.528 | 21.4 | 0.006 |
| Gender | 0.153 | 0.445 | 19.3 | 0.002 |
| Decrease in physical activity | −0.124 | −0.540 | 19.4 | 0.018 |
| Proteinuria | −0.194 | −0.523 | 28.5 | <0.0001 |
| Serum bicarbonate | 0.121 | 0.332 | 11.4 | 0.020 |
Multiple R=0.356.
Variables that did not make the best prediction equation: time of year of extraction; comorbidity index (p<0.10); body mass index; eGFR; uric acid (p<0.10); albumin; C-reactive protein; diabetes; vitamin D analogs; ACE inhibitors/ARA; statins; and xanthine oxidase inhibitors (p=0.05).
Analysis of the multicollinearity of the model showed a condition number of 19.57, i.e. at the limit for considering the model to have severe multicollinearity.
The independent variables that showed significant bilateral correlation were age and physical activity – the greater the age, the less physical activity – (R=0.357; p<0.0001), and age and gender – males younger than females – (R=−0.121; p=0.021).
Analysis of relative weights (Table 2) showed proteinuria to be the variable with the greatest relative importance, followed by age. The sum of both variables contributed almost 50% to multiple R. The other 50% was divided among the following independent variables: decline in physical activity; gender (more vitamin D deficiency in females); and serum bicarbonate concentrations (the lower the bicarbonate levels, the more vitamin D deficiency) (Table 2).
With the best predictive model variables transformed into categoricals, a score was set for prediction of severe 25(OH)D deficiency (concentrations <15ng/ml). Each of the following categories added one point: aged over 70; being female; decreased physical activity; proteinuria >1g/g of creatinine; and serum bicarbonate below 22mEq/l.
The cut-offs for transforming continuous variables into discrete variables were estimated by observing points with greater sensitivity and specificity in receiver operating characteristic curves (Youden index).
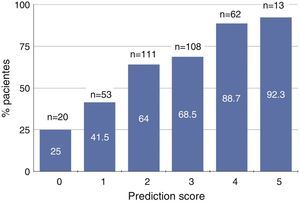
Fig. 1 shows the percentage of patients with severe 25(OH)D deficiency according to the predictive score ranging from 0 to 5. Approximately 90% of patients with scores of 4 or 5 had severe vitamin D deficiency, while only 25% of those with none of these characteristics had vitamin D deficiency (Fig. 1).
Graph showing the percentage of patients with vitamin D deficiency (serum 25-hydroxycholecalciferol levels <15ng/ml) according to predictive score based on age, gender, physical activity, proteinuria and serum bicarbonate. The total number of patients (n) in each scoring subgroup and the percentage with vitamin D deficiency are specified.
The results of this study show that over 80% of patients with advanced CKD have vitamin D deficiency. The predictors of vitamin D concentrations with the most relative importance were proteinuria, age, gender, reduction in physical activity and serum bicarbonate.
A previous study on a large Spanish population, the OSERCE Study,3 describes the main parameters of mineral and bone disorder associated with CKD, including 25(OH)D levels. However, in that study, it was not among their objectives to identify the determinants of 25(OH)D levels, and even if it had been, they would not have been able to. In fact, they concluded that 25(OH)D levels are associated only with PTH and, to a small extent, with proteinuria.3
The design of our study left aside the relationship between 25(OH)D and bone mineral metabolism parameters (probably more effect than cause), and focused on establishing what was or were its determinants. Therefore, unlike the OSERCE study, the predictive information provided by our study on 25(OH)D levels is much more rigorous and thorough, and the analysis included the majority of the known potential determinants. The main original aspect of our study though is the statistical analysis for the “relative importance” weights, i.e. we not only determined which factors were associated qualitatively with 25(OH)D levels, but we also quantified the importance of each factor to explain the association model and compensate for the collinearity inherent to these studies.
Due to the frequent cross-correlation between the variables proposed as determinants of a dependent variable, the resulting models can be inaccurate and the results can lead to confusing interpretations.21 This could be the case when analysing the determinants of 25(OH)D concentrations in CKD.
Use of the relative weights statistical method makes it possible to estimate the relative importance of each independent variable in a prediction model, computing the effect of multicollinearity.21–23
In this study, proteinuria was the variable with the greatest relative importance in the prediction model.
Other studies have also shown an association between the magnitude of proteinuria and the degree of vitamin D deficiency in patients with CKD3,17,18 or in renal transplant recipients.19
In some studies in which proteinuria was not measured, an association was found between vitamin D deficiency and hypoalbuminaemia,4,7,18 or the presence of glomerular diseases that tend to present with severe proteinuria.11,13,25
Since most of the studies on this subject have been cross-sectional, we cannot conclude that there is a causal relationship between proteinuria and vitamin D deficiency.
A high percentage of circulating vitamin D is bound to proteins (DBP and albumin), which are filtered in large quantities through the sick glomeruli and this can lead to significant losses in urine.14 A plausible hypothesis to explain this association would therefore be a vitamin D depletion effect caused by proteinuria.
Further data to support the hypothesis that vitamin D deficiency is associated with protein depletion are the lower 25(OH)D levels in patients on peritoneal dialysis; these patients tend to have higher protein loss than those treated with haemodialysis.26,27
However, there might also be a hypothetical proteinuric effect associated with vitamin D deficiency. Although it has been found that vitamin D analogs (calcitriol, paricalcitol) have a reducing effect on proteinuria, a study has also shown significant reductions in albuminuria after repletion of vitamin D levels with cholecalciferol.28 Proteinuria might therefore not only be a cause but also an effect of vitamin D deficiency, which would reinforce the interpretation of its greater relative importance.
Other aspects such as older age, female gender or decreased physical activity are more predictable factors that contribute to lower vitamin D levels.13,29
One finding difficult to interpret in this study is the significant association, although with less relative importance, between the concentration of serum bicarbonate and 25(OH)D levels.
Acidosis does not cause any effects on the synthesis or degradation of 25(OH)D.30,31 Ketoacidosis32 or ketogenic diets33 could cause reductions in 25(OH)D levels, but this assumption does not seem relevant among our patients.
A common link between acidosis and 25(OH)D reduction in the clinical context of proteinuric kidney disease could be proximal tubule dysfunction.
A more dysfunctional proximal tubule, in addition to possibly causing a more severe acidosis (type 2 renal tubular acidosis) could lead to increased urinary losses of 25(OH)D due to lack of reabsorption of proteins (DBP, albumin), requiring tubular expression of multiligand endocytic receptors, such as megalin and cubilin.34 Vitamin D deficiency has even been identified as direct cause of proximal tubule dysfunction.35 The proximal tubule–proteinuria–vitamin D interaction, though speculative, could help explain these findings.
The time of year the sample for 25(OH)D measurement is taken has been shown to influence the results in some studies.6,7 In very northern continental climates in the northern hemisphere, there is a huge difference between the cold seasons and the hot seasons in terms of the amount of sunlight. In contrast, the climate in Extremadura in Spain is southern continental, with quite sunny winters, which encourage people to go out in the sun, clement springs and autumns, but very hot summers, when it is necessary to shelter from the sun. These aspects may help explain the lack of significant differences in 25(OH)D levels according to the time of year in our patients.
In this study, we found no significant association between low levels of 25(OH)D and statins or ACE inhibitors and/or ARAs. In another recent study20 in a smaller group of patients with predialysis CKD and severe vitamin D deficiency, Yuste et al. found even lower 25(OH)D levels in those treated with statins, and higher levels in those treated with ACE inhibitors or ARAs. In the multivariate analysis of this study, however, the authors did not include proteinuria in the model fit, a fact that might have been of great interest for the interpretation of the results.
In that same study,20 Yuste et al. detected higher 25(OH)D levels in patients treated with allopurinol. In our study, treatment with XO inhibitors (allopurinol or febuxostat) was associated with higher levels of 25(OH)D compared to no such treatment, although this covariate narrowly missed being statistically significant in the multivariate analysis (p=0.05).
There is a relationship between purine metabolism and vitamin D, with an inhibitory effect of uric acid and xanthine on 1-α-hydroxylase having been demonstrated.36–38 However, what role the inhibition of xanthine-oxidase may play in 25(OH)D metabolism is uncertain.
The anti-inflammatory effect of XO inhibitors has been suggested as cause of the higher levels of 25(OH)D.20 In our study, however, the 25(OH)D levels were not correlated with any inflammatory markers, such as C-reactive protein, and nor did those treated with XO inhibitors have lower C-reactive protein concentrations (data not shown). In contrast, we did find that patients treated with XO inhibitors had significantly less proteinuria than those not treated (1.39±1.38 vs. 2.13±2.46g/g creatinine; p=0.005). The collinearity with proteinuria may therefore at least in part explain the association of treatment with XO inhibitors and 25(OH)D levels.
This study has limitations, particularly in relation to the cross-over design, which prevents us from firmly establishing causal inferences.
In conclusion, vitamin D deficiency is very common in CKD, and the magnitude of proteinuria is a factor with high relative importance in predicting reduced 25(OH)D levels. In view of the strong association with proteinuria, any study on the adverse effects of vitamin D deficiency in CKD (e.g. progression of renal failure) should take this relationship into account, since the magnitude of proteinuria and associated factors can also be very important as determinants of the clinical progression and prognosis of this disease.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Caravaca-Fontán F, Gonzales-Candia B, Luna E, Caravaca F. Importancia relativa de los factores determinantes de los niveles séricos de 25-hidroxi-colecalciferol en la enfermedad renal crónica. Nefrologia. 2016;36:510–516.