Renal disease secondary to vasculitis associated with anti-neutrophil cytoplasmic antibodies (ANCA) can lead to chronic renal disease requiring renal replacement therapy. In these patients, kidney transplantation offers excellent long-term rates of allograft and patient survival; consequently, they can be trasplanted when the clinical disease activity has remitted. However, the risk of disease relapses in the renal allograft remains, although at lower rates due to modern immunosuppressive regimes. We describe the case of a male patient with extracapillary glomerulonephritis type III C-ANCA (+) who developed a recurrence in the renal allograft 8 years after transplantation. Intensive immunosupression with plasmapheresis controlled the disease.
La afectación renal de las vasculitis asociadas a anticuerpos anticitoplasma de neutrófilos (ANCA) puede conducir a enfermedad renal crónica con necesidad de tratamiento renal sustitutivo. En estos enfermos el trasplante renal ofrece excelentes tasas de supervivencia del injerto y del receptor a largo plazo, por lo que pueden ser trasplantados cuando la enfermedad está en remisión. Sin embargo, la amenaza de recidivas de la enfermedad en el injerto se mantiene, aunque, con las modernas pautas de inmunosupresión, su incidencia es menor. Presentamos el caso de un varón diagnosticado de glomerulonefritis extracapilar tipo III C-ANCA (+) que desarrolló una recidiva de la enfermedad en el injerto renal 8 años después de ser trasplantado. La intensificación de la inmunosupresión con plasmaféresis consiguió controlar la enfermedad.
Currently, extracapillary glomerulonephritis type III with the demonstration of cytoplasmic antibodies (ANCA) is part of one of the three variants of ANCA-associated vasculitis (AAV) but affecting the kidneys only. The other two would be granulomatosis with polyangiitis (Wegener's granulomatosis) and microscopic polyangiitis.1 These vasculitis are the most frequent cause of rapidly progressive glomerulonephritis. With early diagnosis and the application of therapy, based mainly on steroids and cyclophosphamide, the survival of patients and preservation of renal function is improved. However, more than 20% of these patients, develop end-stage renal disease, requiring renal replacement therapy.2–4
In these patients, renal transplantation is an alternative that provides excellent results, still there are issues that have to be resolved.4–6 First, due to the possibility of recurrence of the disease in the graft,6–11 it is not clear when would be the most appropriate time to include patients on the transplant waiting list. Second, there is no clear agreement on the treatment of recurrences.12
We report the case of a patient with Type III extracapillary glomerulonephritis associated with anti-proteinase 3 ANCA (C-ANCA) who, after 8 years of cadaver kidney graft transplant, had a recurrence of the disease in the graft. Concerning the case, a brief review of the subject has been included.
Clinical caseA 60-year-old male diagnosed in another hospital in 2000 of C-ANCA associated Type III extracapillary glomerulonephritis. He was treated with 5 intravenous bolus of 6-methylprednisolone followed by oral corticosteroids in decreasing doses associated to oral cyclophosphamide (unable to obtain information about exact dosing). At one point, cyclophosphamide was discontinued due to myelotoxicity.
In 2002 the patient was included in a regular haemodialysis program.
In September 2006, he received a cadaver kidney graft in another transplant center. The patient was receiving tacrolimus monotherapy, although we cannot rule out having received some other combination of immunosuppressant therapy. Serum creatinine levels ranged from 1.5 to 1.7mg/dl.
In December 2013, during the implantation of a percutaneous aortic valve in our hospital, a pre and post-intervention clinical evaluation was carried out in our department. At that time his clinical condition was satisfactory, with serum creatinine level of 1.5mg/dl and proteinuria 0.3g/day, with a normal urinary sediment.
In October 2014, due to onset of respiratory symptoms with fever and impaired renal function, the patient requested to be transferred to our hospital for clinical follow-up. He had absence of microbiological and radiologic infections; he improved after empirical treatment with levofloxacin. However in a few days, renal function deteriorated reaching serum creatinine levels of 4mg/dl with proteinuria of 6.8g/day accompanied by haematuria. Determination of C-ANCA was 74.2IU/ml (normal range: 0–5IU ml) and P-ANCA 8.4IU/ml (normal range: 0–6IU/ml). The other autoimmunity parameters (ANA, anti-GBM antibodies, complement, cryoglobulins, etc.) were negative. HIV, HCV, HBV serology, and CMV and BK viremia were also negative.
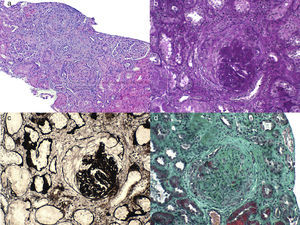
A renal graft biopsy was performed and the most relevant findings were (Fig. 1): 3 out of the 19 evaluable glomeruli had global glomerular sclerosis, and 12 glomeruli had cellular crescents. Some of them had Bowman's capsule disruption causing pseudo-granulomatous inflammatory reaction of mononuclear cells. Two glomeruli had injuries consistent with fibrinoid necrosis. We found tubular necrosis in 15%, tubular atrophy 20% and some casts, plus interstitial infiltration in 25% with some eosinophils and foci of recent interstitial hemorrhage and arteriolar hyalinosis, with some images of wall mucoid degeneration without transmural infiltrate. Immunofluorescence study was negative. Immunohistochemistry for C4d was negative.
Given the evidence of a recurrence of the underlying disease, the patient received 3 intravenous bolus of 500mg of 6-methylprednisolone (the patient was diabetic) on consecutive days, followed by oral prednisone at a dose of 0.5mg/kg/day in descending dosing. Likewise, 8 sessions of plasmapheresis were applied, and we started treatment with mycophenolate mofetil (1g/12h, orally) associated with tacrolimus.
Twelve days after admission, the patient was discharged with a serum creatinine level of 2.9mg/dl. In an outpatient check-up a month later, creatinine level was of 2.3mg/dl with proteinuria of 3.6g/day.
DiscussionTreatment of AAV is based mainly on the association of cyclophosphamide and corticosteroids; this therapy has shown clear efficacy in improving patient survival and preservation of renal function. However, more than 20% of patients develop end stage renal disease requiring renal replacement therapy.2–4 In these patients, renal transplantation is an excellent alternative since it achieves graft survival rates of 90% for 5 years and about 70% for 10 years, with recipient survival of 65% for 10 years and an average survival of 13.4 years. These are a very positive data in comparison to transplanted patients with other types of renal diseases.4–6
The recurrence nature of the disease is a threat to patients. Recent studies compare rates of disease recurrence with different immunosuppressive regimens; Rituximab versus cyclophosphamide associated with corticoids, the reported recurrence rates are 32% with cyclophosphamide and 29% with rituximab at 18 months.13,14
Once patients receive a transplant, the risk of disease recurrence persists. The information we have comes from isolated cases or small series from some centers, still the collection of available data shows that the incidence of recurrence of the disease has decreased as immunosuppression therapy has changed. In 1999, Nachman et al., reviewed 127 published cases and found a recurrence rate of 17%, with 31 months mean time from transplant to recurrence (range from 5 days to 13 years).7 It is important to note that, in these cases the immunosuppressive regime was based largely in cyclosporine A. In consecutive years, with more actual guidelines on immunosuppression, and with the use of antibody induction, corticosteroids, mofetil mycophenolate and tacrolimus, the recurrence rates are apparently lower. These vary depending on the series: 8.6% (Gera et al., 2007),8 4.7% (Little et al., 2009)9 and 8.2% (Geetha et al., 2011).10 It is very likely that the introduction of these drugs to control cellular and humoral immune responses in the transplant has contributed significantly in reducing recurrence rates. In fact, thymoglobulin has been used. It is being suggested the use thymoglobulin or mofetil mycophenolate to control vasculitis activity in certain cases of resistance or intolerance to cyclophosphamide.15,16
This patient experienced a recurrence of the disease in the kidney graft after 8 years transplantation. Although we have been unable to collect complete patient information as he received the transplant at another facility, it seems that vasculitis activity was controlled given that a year earlier, at our center, there was no evidence of disease activity. The reason for initiating an immunosuppressant monotherapy with Tacrolimus was not known. It could have had some involvement in the onset of disease recurrence. Therefore, we venture to point out that in these conditions, any changes in immunosuppressive maintenance regime should be especially weighed and pondered.
Another aspect for consideration would be the moment in which patients should be included in the waiting list for renal transplantation. Although some authors point to the desirability of waiting a year once remission has occurred, as in other related diseases, there seems to be a consensus that patients is ready to receive a transplant once the disease is in complete remission, meaning that there is a total absence of symptoms.8,9,11 Although it should be noted that, in the review by Little et al., renal transplants performed within a year of remission of the disease is a risk factor for mortality (HR 2.3; p=0.04).9
The persistence of detectable ANCA does not mean that the disease is active, in fact, ANCA status is not included either in version 3 of the Birmingham Vasculitis Activity Score (BVAS) or the most recent definition of the European Vasculitis Study Group.17 Guillevin et al. in a recent study described ANCA positivity in 62% (IFA) or 46% (ELISA) of patients already considered in remission.17 Similarly, a significant part of transplant patients, in some series by more than 40%, are ANCA (+) at the time of transplantation.7–9 Moreover, ANCA status at the moment of transplantation doesn’t predict the likelihood of relapse, nor the prognosis of the graft or the patient survival. The type of ANCA (myeloperoxidase or antiproteinase-3) does not predict outcome, although in some work it has been shown an association between the type of ANCA and the development of graft vasculopathy.9
Due to the scarcity of cases and the wide dispersion it is difficult to establish standard treatment guidelines for AAV recurrence in patients with renal transplantation and, therefore, to draw relevant conclusions. Moreover, when deciding the intensity of the treatment, it is important to consider the severity of recurrence using some criteria such as, Birmingham Vasculitis Activity Score.
The medical treatment used in the reported cases is diverse. It includes the reintroduction of cyclophosphamide, plasmapheresis sessions, steroid bolus, intensification of basal immunosuppression, etc.12,18,19 As indicated earlier, we believe that the results cannot be quantified accurately. In recent years, the successful introduction of rituximab, not only for the induction of disease remission but also to control recurrences, opens up new possibilities in AAV17 treatment. Its application has not been tested in transplant patients.
In our case, we chose to intensify immunosuppression through the administration of corticosteroids after 6-methylprednisolone bolus, the administration of mofetil mycophenolate, and maintaining tacrolimus with plasmapheresis sessions. This treatment resulted in a short term control of renal disease.
To resume, we describe the case of a patient with a recurrence of a AAV variant that was controlled, short-term, with the intensification of immunosuppression and plasmapheresis. For patients with end stage renal failure secondary to AAV, renal transplantation provides excellent results and should be considered once remission is established. The possibility of recurrence of the disease exists, although the rate of recurrence is decreasing, probably due to modern immunosuppressive drugs. The addition of Rituximab to more conventional treatments adds optimism to disease control.
Conflict of interestThe authors informed that they had no conflict of interest.
Please cite this article as: García Cosmes P, Fraile Gómez P, Lewczuk K, Rodríguez González M, Ruiz Ferreras E, Tabernero Fernández G. Recidiva de vasculitis asociada a anticuerpos anticitoplasma de neutrófilos en un paciente con trasplante renal. Nefrología. 2016;36:176–180.