Anti-parathyroid treatment initiation and discontinuation are important decisions in chronic haemodialysis (HD) patients, where pill burden is often excessive. The present study aimed to describe secondary hyperparathyroidism (sHPT) drug therapy changes in HD patients.
MethodsRetrospective observational cohort study of incident European HD patients with sHPT who were prescribed calcitriol or alfacalcidol (alpha calcitriol), paricalcitol or cinacalcet.
ResultsTreatment-naïve patients prescribed alpha calcitriol (N=2259), paricalcitol (N=1689) and cinacalcet (N=1245) were considered for analysis. Serum intact parathyroid hormone (iPTH) levels decreased post-initiation with all treatment modalities; serum calcium and phosphate levels increased in response to activated vitamin D derivatives but decreased with cinacalcet. Approximately one-third of alpha calcitriol and paricalcitol patients but less than one-quarter of cinacalcet patients discontinued treatment. Although the three groups had comparable serum iPTH control at the time of treatment discontinuation, they differed in terms of calcium and phosphate levels. Following discontinuation, the evolution of laboratory parameters differed by treatment modality: whilst iPTH increased for all three treatment groups, calcium and phosphate decreased in patients who were being treated with alpha calcitriol and paricalcitol at the time of discontinuation, and increased in those who had been treated with cinacalcet.
ConclusionsIn conditions of daily clinical practice, attaining and maintaining recommended biochemical control of sHPT appears to be more frequently achievable with cinacalcet than with activated vitamin D compounds.
El inicio y la discontinuación del tratamiento antiparatiroideo son decisiones importantes en los pacientes en hemodiálisis crónica (HD) en los que la carga de pastillas es con frecuencia excesiva. El objetivo de este estudio es describir de tratamiento del hiperparatiroidismo secundario (sHPT) en pacientes en HD.
MétodosEstudio de cohorte, observacional retrospectivo de pacientes europeos incidentes en HD con sHPT a quienes se prescribió calcitriol o alfacalcidol (calcitriol-alfa), paricalcitol o cinacalcet.
ResultadosSe incluyeron en el análisis pacientes que recibieron por primera vez calcitriol-alfa (N=2259), paricalcitol (N=1689) y cinacalcet (N=1245). Los valores sericos de hormona paratiroidea intacta (iPTH) disminuyeron tras iniciación con todos los tratamientos; los valores de calcio y fosforo serico se elevaron en respuesta al tratamiento con activadores de vitamina D pero disminuyeron con cinacalcet. Aproximadamente un tercio de los pacientes que recibieron calcitriol alfa y paricalcitol, y menos de una cuarta parte de los de cinacalcet discontinuaron el tratamiento. Aunque los tres grupos tuvieron descensos comparables de iPTH al momento de la interrupción del tratamiento, sin embargo difirieron en los valores de calcio y fosforo serico. Tras la interrupción, la evolución de los parámetros de laboratorio fué diferente según la modalidad de tratamiento: mientras que la iPTH se elevó en las tres modalidades, el calcio y fosforo sericos disminuyeron en los pacientes que estaban siendo tratados con calcitriol-alfa y paricalcitol en el momento de la interrupción y aumentaron en los que lo hacían con cinacalcet.
ConclusionesEn condiciones clínicas que representan la practica diaria, alcanzar y mantener los valores recomendados para el control del sHPT se consigue más frecuentemente con cinacalcet que con compuestos activos de vitamina D.
Secondary hyperparathyroidism (sHPT) occurs early in chronic kidney disease (CKD) and is a major component of the CKD-related mineral and bone disorder (CKD-MBD).1 Major CKD-MBD complications include fractures, cardiovascular disease (CVD), and mortality.2–7
Control of serum calcium, phosphorus and PTH has been associated with reduced mortality risk in haemodialysis patients,8 hence effective sHPT control is important in these patients. Reduced persistence, compliance, and/or adherence with oral medications are a common problem, especially in CKD patients,9 with non-adherence rates of 3–80% reported.10–12 Non-adherence was associated with increased mortality in the dialysis population,13 highlighting a potential unmet need among patients who do not persist with sHPT therapies. Clinical trial data,14 where persistence-corrected analyses revealed more beneficial effects associated with cinacalcet (Sensipar®/Mimpara®) prescribing than uncorrected analyses, support this hypothesis.
The current study, conducted in European patients initiating haemodialysis (HD) treatment, aimed to describe separately the characteristics of patients who initiated long-term sHPT therapies with either prescribed activated vitamin D sterols (AVDs) or cinacalcet, the conditions and reasons for non-persistence and the consequences of initiation and non-persistence, respectively, for the control of serum biochemistry parameters.
MethodsData sourceA retrospective cohort study was conducted using the second cohort of the Analysing Data, Recognising Excellence and Optimising Outcomes research initiative (AROii).15,16 Anonymised patient-level medical history data, longitudinal laboratory, dialysis and medication data, plus ICD-10 coded hospitalisation and death data17 are supplied quarterly. All ethical and regulatory obligations concerning patient data in this study were met locally and informed consent was obtained from all patients.
Study populationThe study population included incident (<183 days dialysis vintage, i.e. the time since dialysis initiation) adult patients (≥18 years) receiving haemodialysis at >300 Fresenius Medical Care (EU-FMC) facilities in 14 European countries and Turkey from 2007 to 2009.16 Patients on short-term haemodialysis treatment (<10 contiguous dialysis sessions), with a history of peritoneal dialysis or kidney transplantation were excluded. Patients with <90 days of follow-up or a parathyroidectomy history of up to/including the first 90 days of follow-up were also excluded.
Patients’ first haemodialysis session defined study start and follow-up start. Three sub-populations, comprising patients prescribed calcitriol/alfacalcidol (henceforth calcitriol-alfa), paricalcitol or cinacalcet, were created. Within each treatment sub-population, patients were excluded if they were prescribed that treatment before study start or during the first 90 days of follow-up (to exclude prevalent users), if the first prescription duration was less than 15 days with no further treatment prescriptions (to effectively capture therapeutic effects) or if the first AVDs and cinacalcet prescriptions were on the same day (to distinguish the therapy effects).
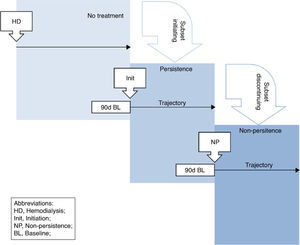
Initiation, discontinuation definitionsInitiation was defined by patients’ first prescription start date for each treatment (henceforth initiation index date; Fig. 1). If patients’ first prescription was <15 days long and the gap between prescription end and the next was >15 days then the next prescription was considered for initiation (with the same rules applied) and so forth. Treatment discontinuation was defined as the time point (henceforth discontinuation index date) beyond which there were ≥45 consecutive prescription-free days. Patients were considered persistent until discontinuation.
The 90 days before each index date constituted baseline. Patients were followed-up for 12 months post-initiation/discontinuation or until end of follow-up (first occurrence of parathyroidectomy, renal transplantation, death or loss-to-follow-up (>45 days without EU-FMC dialysis) or end of study follow-up (31 December 2013)).
Serum biochemistrySerum concentrations of total calcium (henceforth calcium), phosphate and parathyroid hormone (PTH) were measured locally. Serum ‘intact’ PTH (iPTH) values were determined locally with different assays. Uncorrected iPTH concentrations were used.
Statistical analysisStatistical analyses were conducted in SAS (version 9.2; SAS, Cary, NC, USA) and reproduced independently. Initially, baseline characteristics of initiating and discontinuing patients were presented. Data were categorised into quartiles or biologically relevant groups. Continuous variables were described using mean, standard deviation, median, 25th and 75th percentile values; categorical data were reported as counts and percentages.
Mean calcium, phosphate and iPTH values were determined at baseline and each quarter during the 12 months post-initiation/discontinuation or end of follow-up (whichever occurred first), with the average change from baseline also presented. Median percentage change from baseline was presented to reflect the skewed distribution of these values.
To describe treatment persistence patterns, Kaplan–Meier estimates for time-to-discontinuation were computed and graphically displayed. Quarterly post-initiation Kaplan–Meier persistence probabilities were estimated with 95% confidence intervals (CI).
Sensitivity/additional analysesA sensitivity discontinuation definition was applied to investigate its effect on the findings: discontinuation was defined as the start of the 90-day interval post-initiation where the proportion of prescribed days over the interval was <67% (i.e. <60/90 days coverage).
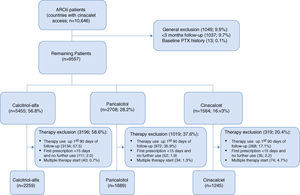
ResultsStudy populationBetween 01 January 2007 and 31 December 2009, 10,646 chronic haemodialysis patients treated in 310 EU-FMC facilities in 14 European countries and in Turkey were recruited (Fig. 2). Of these, 1049 patients (9.9%) were excluded because their follow-up was <90 days (1037; 9.7%) or for a parathyroidectomy history (13; 0.1%), leaving 9597 patients for the treatment-specific analyses.
Prescribed drugs for sHPT treatment were calcitriol-alfa (5455 patients; 56.8%); paricalcitol (2708 patients; 28.2%); and cinacalcet (1564; 16.3%). Among these, 57.5%, 35.9% and 17.1%, respectively, were prescribed treatments in the first 90 days of HD; most treated patients were excluded for this reason. Additionally, cinacalcet patients were more often excluded due to multiple therapy starts. Applying all the exclusion criteria, 2259 calcitriol-alfa, 1689 paricalcitol and 1245 cinacalcet patients remained. Most initial paricalcitol prescriptions (1459; 86.4%) were administered intravenously; calcitriol-alfa and cinacalcet are oral medications. Most cinacalcet patients (996; 80.0%) had previously initiated AVD.
Patient characteristics by treatment typeBaseline characteristics at initiationLarge geographical differences were observed in patients initiating calcitriol-alfa, paricalcitol and cinacalcet treatment (Table 1), probably related to differing drug access and reimbursement policies. Similarly, the decreasing trend for initiating treatment after the KDIGO guideline2 publication may reflect differing marketing authorisation (calcitriol-alfa, then paricalcitol, then cinacalcet). No major clinical history differences were apparent.
Characteristics of patients in the 90 days prior to initiating and discontinuing CKD-MBD treatment.
| Parameter | Initiation | Discontinuation | ||||
|---|---|---|---|---|---|---|
| Calcitriol-alfaa | Paricalcitol | Cinacalcet | Calcitriol-alfa | Paricalcitol | Cinacalcet | |
| (N=2259) | (N=1689) | (N=1245) | (N=755) | (N=540) | (N=290) | |
| Age | ||||||
| Mean±SD | 64.3±14.5 | 65.2±14.8 | 63.4±15.4 | 63.3±14.3 | 64.2±14.8 | 65.3±15.1 |
| Gender | ||||||
| Male | 1304 (57.7) | 1026 (60.7) | 709 (56.9) | 416 (55.1) | 325 (60.2) | 158 (54.5) |
| Female | 955 (42.3) | 663 (39.3) | 536 (43.1) | 339 (44.9) | 215 (39.8) | 132 (45.5) |
| Geographical area | ||||||
| Eastern Europed | 950 (42.1) | 436 (25.8) | 121 (9.7) | 450 (59.6) | 181 (33.5) | 19 (6.6) |
| Western Europee | 479 (21.2) | 130 (7.7) | 130 (10.4) | 76 (10.1) | 34 (6.3) | 22 (7.6) |
| Iberian peninsulaf | 830 (36.7) | 1123 (66.5) | 994 (79.8) | 229 (30.3) | 325 (60.2) | 249 (85.9) |
| Period pre-KDIGOb | ||||||
| Yes | 743 (32.9) | 503 (29.8) | 276 (22.2) | 170 (22.5) | 96 (17.8) | 44 (15.2) |
| No | 1516 (67.1) | 1186 (70.2) | 969 (77.8) | 585 (77.5) | 444 (82.2) | 246 (84.8) |
| Smoking status | ||||||
| Current | 179 (7.9) | 167 (9.9) | 131 (10.5) | 64 (8.5) | 59 (10.9) | 27 (9.3) |
| Former | 356 (15.8) | 293 (17.3) | 164 (13.2) | 131 (17.4) | 95 (17.6) | 36 (12.4) |
| Non-smoker | 1016 (45.0) | 634 (37.5) | 423 (34.0) | 380 (50.3) | 240 (44.4) | 102 (35.2) |
| Missing | 708 (31.3) | 595 (35.2) | 527 (42.3) | 180 (23.8) | 146 (27.0) | 125 (43.1) |
| BMI (kg/m2) | ||||||
| <18.5 | 70 (3.1) | 37 (2.2) | 23 (1.8) | 21 (2.8) | 12 (2.2) | 5 (1.7) |
| 18.5–<25 | 795 (35.2) | 596 (35.3) | 404 (32.4) | 259 (34.3) | 188 (34.8) | 86 (29.7) |
| 25–<30 | 677 (30.0) | 554 (32.8) | 438 (35.2) | 240 (31.8) | 187 (34.6) | 107 (36.9) |
| ≥30 | 440 (19.5) | 340 (20.1) | 252 (20.2) | 161 (21.3) | 100 (18.5) | 64 (22.1) |
| Missing | 277 (12.3) | 162 (9.6) | 128 (10.3) | 74 (9.8) | 53 (9.8) | 28 (9.7) |
| Blood pressure (mmHg)c | ||||||
| <120 | 435 (19.3) | 374 (22.1) | 209 (16.8) | 156 (20.7) | 136 (25.2) | 78 (26.9) |
| 120–<130 | 459 (20.3) | 360 (21.3) | 237 (19.0) | 142 (18.8) | 104 (19.3) | 44 (15.2) |
| 130–<140 | 483 (21.4) | 337 (20.0) | 266 (21.4) | 170 (22.5) | 122 (22.6) | 67 (23.1) |
| 140–<160 | 677 (30.0) | 473 (28.0) | 409 (32.9) | 216 (28.6) | 144 (26.7) | 78 (26.9) |
| ≥160 | 205 (9.1) | 145 (8.6) | 124 (10.0) | 71 (9.4) | 34 (6.3) | 23 (7.9) |
| Clinical history | ||||||
| Hospitalisation | 312 (13.8) | 206 (12.2) | 121 (9.7) | 92 (12.2) | 71 (13.1) | 31 (10.7) |
| Diabetes | 809 (35.8) | 598 (35.4) | 385 (30.9) | 266 (35.2) | 176 (32.6) | 89 (30.7) |
| Cancer | 154 (6.8) | 131 (7.8) | 116 (9.3) | 47 (6.2) | 46 (8.5) | 35 (12.1) |
| Cardiovascular disease | 812 (35.9) | 740 (43.8) | 569 (45.7) | 271 (35.9) | 238 (44.1) | 151 (52.1) |
| Fracture | 52 (2.3) | 61 (3.6) | 59 (4.7) | 24 (3.2) | 17 (3.1) | 19 (6.6) |
| CKD aetiology | ||||||
| Hypertension/vascular | 373 (16.5) | 319 (18.9) | 216 (17.3) | 144 (19.1) | 98 (18.1) | 56 (19.3) |
| Glomerulonephritis | 214 (9.5) | 156 (9.2) | 154 (12.4) | 72 (9.5) | 47 (8.7) | 36 (12.4) |
| Diabetes | 516 (22.8) | 400 (23.7) | 235 (18.9) | 174 (23.0) | 106 (19.6) | 61 (21.0) |
| Tubulo-interstitial | 228 (10.1) | 158 (9.4) | 145 (11.6) | 82 (10.9) | 52 (9.6) | 31 (10.7) |
| Polycystic kidney disease | 114 (5.0) | 107 (6.3) | 88 (7.1) | 44 (5.8) | 36 (6.7) | 19 (6.6) |
| Invalid/missing/unknown | 814 (36.0) | 549 (32.5) | 407 (32.7) | 239 (31.7) | 201 (37.2) | 87 (30.0) |
| Dialysis vintage (days) | ||||||
| Mean±SD | 575.8±434.1 | 664.1±477.0 | 758.8±490.3 | 701.5±401.4 | 800.4±432.8 | 885.8±449.5 |
| Dialysate calcium concentration (mEq/L) | ||||||
| Mean±SD | 2.871±0.289 | 2.825±0.251 | 2.760±0.255 | 2.909±0.295 | 2.820±0.258 | 2.832±0.258 |
| <3.0 | 722 (32.0) | 670 (39.7) | 631 (50.7) | 232 (30.7) | 233 (43.1) | 116 (40.0) |
| ≥3.0 | 1316 (58.3) | 962 (57.0) | 563 (45.2) | 476 (63.0) | 294 (54.4) | 168 (57.9) |
| Missing | 221 (9.8) | 57 (3.4) | 51 (4.1) | 47 (6.2) | 13 (2.4) | 6 (2.1) |
| Catheter vascular access | ||||||
| Yes | 587 (26.0) | 477 (28.2) | 324 (26.0) | 149 (19.7) | 125 (23.1) | 77 (26.6) |
| No | 1511 (66.9) | 1135 (67.2) | 878 (70.5) | 558 (73.9) | 391 (72.4) | 206 (71.0) |
| Missing | 161 (7.1) | 77 (4.6) | 43 (3.5) | 48 (6.4) | 24 (4.4) | 7 (2.4) |
| Net ultrafiltration (L) | ||||||
| Mean±SD | 1.97±0.83 | 2.07±0.80 | 2.05±0.78 | 2.03±0.85 | 2.09±0.84 | 2.03±0.79 |
| Laboratory parametersg | ||||||
| Blood hemoglobin (g/dL) | 11.51±1.39 | 11.77±1.38 | 11.87±1.31 | 11.48±1.35 | 11.81±1.37 | 11.59±1.46 |
| Ferritin (μg/L) | 479.8±286.8 | 469.6±291.9 | 441.0±267.9 | 530.6±293.4 | 502.6±295.7 | 455.6±266.2 |
| Serum CRP (mg/L)h | 5.3 [2.1,13.6] | 5.4 [2.0,13.3] | 4.9 [1.9,10.5] | 5.4 [2.2,12.5] | 5 [1.6,14.1] | 4.9 [2.0,11.2] |
| Serum albumin (g/L) | 38.6±4.7 | 39.1±4.4 | 39.7±4.4 | 39.6±4.3 | 39.6±3.8 | 38.9±4.6 |
| Serum creatinine (μmol/L) | 661.5±213.0 | 664.6±196.3 | 728.7±207.5 | 697.7±211.5 | 691.3±184.7 | 706.8±203.2 |
| Serum total calcium (mmol/L) | 2.17±0.19 | 2.17±0.17 | 2.28±0.19 | 2.26±0.21 | 2.33±0.22 | 2.14±0.21 |
| Serum phosphate (mmol/L) | 1.45±0.43 | 1.48±0.40 | 1.73±0.48 | 1.65±0.51 | 1.66±0.52 | 1.45±0.47 |
| Serum iPTH (pg/mL) | 419.3±336.7 | 499.8±287.1 | 663.4±371.4 | 296.4±295 | 276.4±285 | 313.1±387.7 |
| Serum calcium (mmol/L) | ||||||
| <2.10 | 708 (31.3) | 491 (29.1) | 180 (14.5) | 131 (17.4) | 57 (10.6) | 112 (38.6) |
| ≥2.10–≤2.37 | 1219 (54.0) | 989 (58.6) | 674 (54.1) | 421 (55.8) | 280 (51.9) | 133 (45.9) |
| >2.37 | 261 (11.6) | 192 (11.4) | 376 (30.2) | 199 (26.4) | 200 (37.0) | 42 (14.5) |
| Missing | 71 (3.1) | 17 (1.0) | 15 (1.2) | 4 (0.5) | 3 (0.6) | 3 (1.0) |
| Serum phosphate (mmol/L) | ||||||
| <1.13 | 475 (21.0) | 272 (16.1) | 102 (8.2) | 120 (15.9) | 80 (14.8) | 63 (21.7) |
| ≥1.13–≤1.78 | 1345 (59.5) | 1113 (65.9) | 626 (50.3) | 343 (45.4) | 248 (45.9) | 167 (57.6) |
| >1.78 | 419 (18.5) | 292 (17.3) | 512 (41.1) | 292 (38.7) | 211 (39.1) | 59 (20.3) |
| Missing | 20 (0.9) | 12 (0.7) | 5 (0.4) | 0 (0.0) | 1 (0.2) | 1 (0.3) |
| Serum iPTH (pg/mL) | ||||||
| <150 | 225 (10.0) | 77 (4.6) | 40 (3.2) | 263 (34.8) | 215 (39.8) | 133 (45.9) |
| ≥150–<300 | 462 (20.5) | 246 (14.6) | 65 (5.2) | 174 (23.0) | 139 (25.7) | 69 (23.8) |
| ≥300–<600 | 882 (39.0) | 855 (50.6) | 502 (40.3) | 145 (19.2) | 127 (23.5) | 41 (14.1) |
| ≥600 | 302 (13.4) | 414 (24.5) | 562 (45.1) | 87 (11.5) | 49 (9.1) | 44 (15.2) |
| Missing | 388 (17.2) | 97 (5.7) | 76 (6.1) | 86 (11.4) | 10 (1.9) | 3 (1.0) |
| Concomitant medication | ||||||
| Calcitriol/alfacalcidol | – | 613 (36.3) | 345 (27.7) | – | 84 (15.6) | 72 (24.8) |
| Paricalcitol | 177 (7.8) | – | 500 (40.2) | 17 (2.3) | – | 124 (42.8) |
| Cinacalcet | 136 (6.0) | 231 (13.7) | – | 37 (4.9) | 117 (21.7) | – |
| Cardiovascular medicationi | 1451 (64.2) | 1277 (75.6) | 1004 (80.6) | 505 (66.9) | 415 (76.9) | 237 (81.7) |
| Phosphate binder use | ||||||
| None | 1107 (49.0) | 503 (29.8) | 296 (23.8) | 266 (35.2) | 106 (19.6) | 59 (20.3) |
| Calcium-based | 872 (38.6) | 727 (43.0) | 433 (34.8) | 405 (53.6) | 243 (45.0) | 126 (43.4) |
| Non calcium-based | 280 (12.4) | 459 (27.2) | 516 (41.4) | 84 (11.1) | 191 (35.4) | 105 (36.2) |
NB, all data are n (%) unless stated otherwise.
Cinacalcet patients’ dialysis vintage exceeded paricalcitol patients’ vintage, which in turn exceeded calcitriol-alfa patients’. Lower dialysate calcium concentrations (<3.0mEq/L) were utilised for paricalcitol or calcitriol-alfa initiators than for cinacalcet initiators; other dialysis parameters were comparable.
The initial extent of CKD-MBD, based on mean serum biochemistry, showed higher levels of calcium (2.28 [95% CI: 2.27,2.29] mmol/L), phosphate (1.73 [95% CI: 1.71,1.76] mmol/L) and iPTH (663 [95% CI: 642,685] pg/mL) for cinacalcet patients when compared to paricalcitol (calcium 2.17 [95% CI: 2.16,2.18] mmol/L, phosphate 1.48 [95% CI: 1.46,1.50] mmol/L and iPTH 500 [95% CI: 486,514] pg/mL) and calcitriol-alfa (calcium 2.17 [95% CI: 2.16,2.17] mmol/L, phosphate 1.45 [95% CI: 1.43,1.47] mmol/L and iPTH 419 [95% CI: 404,435] pg/mL) patients, respectively. No clinical differences between paricalcitol and calcitriol-alfa were observed with regard to serum biochemistry-based CKD-MBD severity.
For baseline concomitant therapies, calcitriol-alfa patients were prescribed phosphate binders less frequently; compared with calcitriol-alfa patients, the proportion of patients prescribed non-calcium-based phosphate binders was more than double for paricalcitol patients and nearly fourfold higher for cinacalcet patients. Cinacalcet patients were often prescribed concomitant AVDs. Cardiovascular medicine prescribing followed a similar trend as non-calcium-based phosphate binder use.
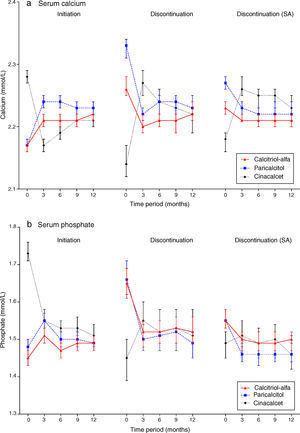
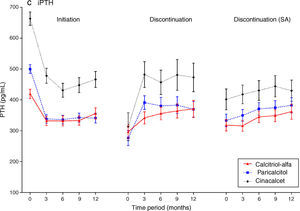
Laboratory trends post-initiationPost-initiation trends in CKD-MBD biochemical markers are described in Fig. 3 (supplementary underlying data and additional graphs are available on-line). Commensurate with the increased pre-initiation levels described above, the observed reduction – quantified as mean change and median percent change from baseline – in serum iPTH for calcitriol-alfa patients (−78.7 [95% CI: −90.4,−66.9] pg/mL; −19.2% [95% CI: −22.1%,−16.4%]) was less marked than in paricalcitol (−165 [95% CI: −180,−151] pg/mL; −31.9% [95% CI: −35.4%,−29.4%]) or cinacalcet (−189 [95% CI: −210,−168] pg/mL; −30.5% [95% CI: −33.8%,−26.7%]) patients in the 3 months post-initiation (mean absolute achieved values of 332, 338 and 478pg/mL, respectively). Mean iPTH levels were largely maintained for calcitriol-alfa and paricalcitol patients for the remaining of the first year; for cinacalcet patients levels continued to decline from 3 (478pg/mL) to 6 months (431pg/mL) before increasing to levels similar to those observed at 3 months post-initiation (466pg/mL).
Mean serum calcium levels increased in the first 3 months for calcitriol-alfa (+0.05 [95% CI: 0.04,0.05] mmol/L; +1.4% [95% CI: 1.2%,2.1%]) and paricalcitol (+0.07 [95% CI: 0.06,0.08] mmol/L; +2.5% [95% CI: 2.3%,3.3%]) patients and were then maintained for the rest of the first year. Increases in mean calcium were higher for paricalcitol than for calcitriol-alfa patients in the 6 months post-initiation. For cinacalcet initiators, mean calcium dropped in the first 3 months (−0.11 [95% CI: −0.12,−0.10] mmol/L; −4.7% [95% CI: −5.2%,−4.2%]) and remained below the pre-initiation levels despite subsequent increases.
Increases in mean serum phosphate in the first 3 months post-initiation were observed with calcitriol-alfa (+0.05 [95% CI: 0.04,0.07] mmol/L; +3.6% [95% CI: 2.5%,5.0%]) and paricalcitol (+0.07 [95% CI: 0.05,0.09] mmol/L; +4.9% [95% CI: 2.7%,6.7%]). Mean phosphate decreased in cinacalcet initiators over the same period; the decrease was more substantial than for calcium (−0.18 [95% CI: −0.21,−0.15] mmol/L; −10.6% [95% CI: −12.3%,−9.3%]) and continued to 12 months.
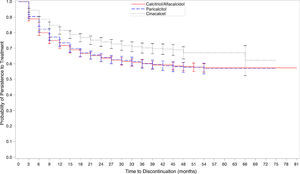
Patterns of discontinuation and associated patient characteristicsThe proportion of calcitriol-alfa (755/2259; 33.4%) and paricalcitol (540/1689; 32.0%) patients discontinuing treatment (main discontinuation definition: >45 day prescription gap) was higher than for cinacalcet patients (290/1245; 23.3%). The median [Q1,Q3] time to discontinuation was 4.4 [95% CI: 2.4,10.3], 5.2 [95% CI: 2.8,11.6] and 6.6 [95% CI: 3.2,13.0] months, respectively. Superior cinacalcet persistence became apparent after 12 months and continued for the study duration (Fig. 4). No separation was observed between calcitriol-alfa and paricalcitol treatment persistence.
No age differences were apparent in discontinuing patients’ baseline characteristics (Table 1); females were consistently under-represented. Eastern European patients were overrepresented amongst the calcitriol-alfa discontinuers whilst Iberian Peninsula patients were over-represented amongst discontinuing paricalcitol and cinacalcet patients.
Among discontinuers, more paricalcitol and cinacalcet patients’ pre-dialysis systolic blood pressure was <120mmHg and a cancer, CVD or fracture history was more common amongst cinacalcet patients. Haemodialysis technique parameters also differed: dialysate calcium concentrations were lower for paricalcitol and cinacalcet patients whilst vascular catheterisation was more prevalent.
Major biochemical differences were observed for CKD-MBD parameters. Whilst mean pre-discontinuation iPTH levels were more similar for all three therapies (296 [95% CI: 274,319] pg/mL, 276 [95% CI: 252,301] and 313 [95% CI: 268,358] pg/mL, respectively, for calcitriol-alfa, paricalcitol and cinacalcet), pre-discontinuation calcium and phosphate levels differed substantially. Mean pre-discontinuation calcium was higher in paricalcitol (2.33 [95% CI: 2.31,2.34] mmol/L) than in calcitriol-alfa (2.26 [95% CI: 2.25,2.28] mmol/L) patients, which in turn exceeded that in cinacalcet patients (2.14 [95% CI: 2.12,2.17] mmol/L). Mean pre-discontinuation phosphate was similarly elevated in calcitriol-alfa (1.65 [95% CI: 1.61,1.69] mmol/L) and paricalcitol (1.66 [95% CI: 1.62,1.71] mmol/L) patients compared to cinacalcet patients (1.45 [95% CI: 1.39,1.50] mmol/L).
Few discontinuing calcitriol-alfa or paricalcitol patients were prescribed paricalcitol and calcitriol-alfa, respectively, hence multiple AVDs prescribing was rare. Phosphate binder prescribing was markedly higher amongst paricalcitol and cinacalcet patients, with the proportion of patients prescribed non-calcium-based phosphate binders also higher in these patient groups.
Laboratory trends post-discontinuationFollowing discontinuation (main definition; Fig. 3), mean calcium levels decreased in calcitriol-alfa (−0.06 [95% CI: −0.07,−0.04] mmol/L; −2.2% [95% CI: −2.7%,−1.2%]) and paricalcitol (−0.11 [95% CI: −0.12,−0.09] mmol/L; −3.4% [95% CI: −4.4%,−2.4%]) patients within the first 3 months before stabilising. In cinacalcet patients mean calcium levels increased markedly initially (+0.13 [95% CI: 0.10,0.16] mmol/L; +6.0% [95% CI: 4.4%,7.2%]) but subsequently decreased towards levels comparable with calcitriol-alfa and paricalcitol patients at 12 months. Mean phosphate patterns were similar to calcium, with rapid observed decreases in calcitriol-alfa (−0.13 [95% CI: −0.17,−0.10] mmol/L; −7.6% [95% CI: −9.6%,−5.4%]) and paricalcitol (−0.16 [95% CI: −0.20,−0.12] mmol/L; −9.3% [95% CI: −11.7%,−6.4%]) but a slight increase in cinacalcet discontinuers (+0.10 [95% CI: 0.05,0.15] mmol/L; +6.0% [95% CI: 2.2%,10.3%]), with relatively stable levels after 3 months for all three treatment groups. Post-discontinuation mean iPTH levels increased rapidly in paricalcitol (+115 [95% CI: 91,138] pg/mL; +61.2% [95% CI: 45.8%,73.5%]) and cinacalcet (+163 [95% CI: 120,207] pg/mL; +93.0% [95% CI: 69.2%,129.6%]) patients before stabilising; the observed increase for calcitriol-alfa patients was more gradual (+49 [95% CI: 29,69] pg/mL; +32.0% [95% CI: 23.8,41.2]) at 3 months and continued to 12 months.
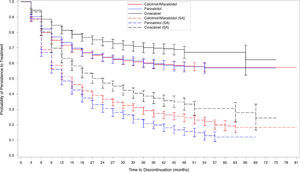
Discontinuation sensitivity analysisApplying the sensitivity analysis discontinuation definition, higher levels of discontinuation were observed for all three treatment groups (1372, 60.7%; 1096, 64.5%; and 608, 48.8% for calcitriol-alfa, paricalcitol and cinacalcet, respectively). The temporal persistence patterns differed markedly (Fig. 5), with lower observed persistence for all three therapies from 6 months. Cinacalcet persistence remained higher for most of follow-up (over 4 years), whilst lower levels of paricalcitol persistence was observed from 2 years.
As the discontinuation findings were definition-sensitive, trajectory analyses were repeated using the new definition (Fig. 3). The observed patterns were similar for serum calcium and phosphate, albeit with less extreme baseline values. The serum iPTH patterns were consistently different, however, with prolonged increases over the year.
DiscussionIn this large European haemodialysis patient cohort, more patients initiated – as evidenced by prescriptions – calcitriol-alfa than paricalcitol, with fewest cinacalcet initiations observed. Major geographical prescribing practice differences were observed, reflecting drug reimbursement policies and the availability/application of national and international guidelines. Physician and centre habits might also influence the observed baseline differences in sHPT treatment, with sHPT severity and serum calcium in particular dictating prescribing patterns. High PTH, phosphorus and, to a lesser extent, calcium were drivers for cinacalcet prescription in approved countries.
The observed PTH reductions with the AVDs might be mediated in part by associated increases in serum calcium. The increases in serum calcium with paricalcitol accord with previous observational research18 but not clinical trial data19,20; the stricter inclusion/exclusion criteria employed in the RCTs might explain the discrepancy. The reduction in all three parameters among cinacalcet initiators is consistent with previous research.21–24
Whilst reasonably good real-life control of serum iPTH levels was achieved in most treated patients in this study, this was not the case for control of serum calcium and phosphate, which was more often outside KDOQI target ranges (and to some extent also outside KDIGO ranges) in AVD-treated patients. The less favourable pre-treatment control in the cinacalcet group than in the AVDs groups was probably due to the secondary introduction of cinacalcet in those patients resistant to AVDs treatment. Attainment of all three mineral metabolism targets was poor, even with treatment. This is a near-universal observation, reflecting the real-life challenges in this regard, where correcting one parameter often represents a trade-off against control of another.
Whilst post-initiation laboratory trajectories have been described previously, as outlined above, this is possibly the first study to describe in detail non-persistence to sHPT treatments and the evolution of laboratory parameters post-discontinuation. The greater observed persistence to cinacalcet compared to AVDs might relate to clinician preference or the lack of a suitable alternative in patients uncontrolled on AVDs treatment alone. Laboratory biochemistry post-discontinuation largely mirrored that observed for initiation and differed by treatment modality: whilst iPTH increased for all three treatment groups, calcium and phosphate decreased for calcitriol-alfa and paricalcitol patients and increased for cinacalcet patients. When an alternative prescription-coverage threshold definition for discontinuation was applied, non-persistence rates increased for all three treatment groups and laboratory trajectories were more conservative. We hypothesise that our main definition may represent definitive treatment drop-outs which occur infrequently, whilst our sensitivity definition captures treatment adaptations which occur more frequently.
Drug discontinuation may be patient- or physician-led and it was impossible to determine in this study which were patient decisions, based on drug intolerance or pill fatigue, and which were medical decisions based on overcorrection of PTH levels (all treatment groups), undesirable increases in calcium and phosphate levels (mostly with AVDs) or undesirable decreases in calcium levels (with cinacalcet). Discontinuation may also be driven by laboratory goal-attainment, suggesting that some clinicians do not view sHPT as a long-term chronic disorder requiring continuous therapy. Most initial paricalcitol prescriptions were administered intravenously, affording a greater clinical influence on treatment decisions for this group. Nevertheless, the pattern of paricalcitol non-persistence (main definition) was no different from the exclusively oral calcitriol-alfa group, suggesting that, for AVDs at least, treatment discontinuation was mainly clinician-led.
Other methodological aspects require consideration. This study utilised data from a single private dialysis provider, hence findings might partially reflect internal policies, limiting generalizability. We assume 100% persistence/non-persistence when this is unlikely. Patients could contribute to each treatment sub-population and/or be on multiple therapies at once and this is not captured/reflected, increasing the potential for confounding. Accordingly, these results could be considered within the framework of ‘assessing the effect of the initial intention of a single treatment (or its withdrawal)’. Our 45-day rule for discontinuation, whilst internally consistent within AROii, is arbitrary and other time periods might have been more appropriate (others have advocated for data-driven definitions based on prescription length25). Our descriptive analytical approach, where statistical modelling was not utilised, does not take into consideration characteristics (observed or otherwise) which could confound the relationship between patients’ biochemistry and treatment initiation/discontinuation and could explain, to an extent, the clinical differences observed between the treatment groups. Similarly, we focused on total treatment populations when sub-group analyses might have been more revealing. For example, the different prescribing pattern in the Iberian Peninsula – especially with regard to cinacalcet – might provide insights into biochemical control which could inform healthcare decision-making by clinicians, policy makers, payers, etc., but this was beyond the scope of the study. Finally, we reported but did not impute missing data which may be important if, for example, less frequent iPTH measurement is indicative of poorer clinical care which in turn is reflected in poorer control of calcium and/or phosphorus.
In conclusion, sHPT biochemistry parameters dictated treatment initiation and discontinuation within the confines of differing geographical treatment patterns. Clinicians appear to adhere to treatment guidelines, although goal achievement is poor. In real-life conditions, better biological control of sHPT appears to be more easily achievable with cinacalcet than with AVDs, where iPTH response might be mediated in part by serum calcium.
FundingThe ARO CKD Research Initiative is a joint observational research commitment from Amgen and Fresenius Medical Care, fully funded by Amgen (Europe) GmbH, Zug, Switzerland.
The sponsors were responsible for data collection (FMC) and data management (FMC, Amgen), provided resources for statistical and epidemiological analysis, and participated in the interpretation of data and preparation of the manuscript (Amgen).
Every step of development of the project, from design and scientific conduct of the study, through interpretation of the data, to preparation, review, and approval of the manuscript, was led by authors who are also members of the ARO Steering Committee. Results and their interpretations were discussed by all members of the ARO Steering Committee at plenary meetings twice a year.
DisclosuresA.L. de F. reports having received Advisor/Consultant fees from Amgen, Fresenius; speaker fees from Abbott, Amgen and Fresenius.
J.F. reports having received Advisor/Consultant fees from Abbott, Amgen, Chugai, Genzyme and Vifor; speaker fees from Abbott, Amgen, FMC, Genzyme and Mitsubishi.
F.K. reports having received Advisor/Consultant fees from Amgen. T.B.D. reports having received Advisor/Consultant fees from Abbott, Amgen, Baxter, FMC, Genzyme, KAI Pharmaceuticals, Kirin, Theraclion, and Vifor; speaker fees from Abbott, Amgen, Chugai, Genzyme, Kirin, and Vifor; and grant/research support from Amgen, Baxter, and Shire.
D.C.W. reports having received research funding from Abbott, Genzyme and AstraZeneca, honoraria from Amgen, Abbott, Fresenius, Janssen, Otsuka, Shire and Vifor.
I.G. is a contractor for Amgen. I.A.G. and M.F. are full-time employees of Amgen who may own stock and/or stock options in Amgen. D.M. is a full-time employee of Fresenius Medical Care.
Prof. P. Aljama, MD, Reina Sofia University Hospital, Cordoba, Spain.
Prof. S. Anker, MD, Dept of Innovative Clinical Trials, University Medical Centre, Göttingen, Germany.
Prof. T. B. Drueke, MD, Inserm Unit 1088, Université de Picardie, Amiens, France.
Prof. K.-U. Eckardt (co-chair), MD, University of Erlangen- Nürnberg, Germany.
Prof. J. Floege (chair), MD, RWTH University of Aachen, Aachen, Germany.
Prof. A. de Francisco, MD, Hospital Universitario Valdecilla, Universidad de Cantabria, Santander, Spain.
Prof. F. Kronenberg, MD, Medical University of Innsbruck, Innsbruck, Austria.
Prof. I. C. Macdougall, MD, King's College Hospital, London, UK.
Prof. J. Malyszko, MD, Medical University of Bialystok, Poland.
Prof. G. Schernthaner, MD, Rudolfstiftung Hospital, Vienna, Austria.
Prof. P. Stenvinkel, MD, Karolinska Institutet, Stockholm, Sweden.
Prof. D. C. Wheeler, MD, Division of Medicine, University College London, London, UK.
Dr. B. Molemans, MD, Amgen Europe GmbH, Zug, Switzerland.
Dr. B. Fouqueray, MD, Amgen Europe GmbH, Zug, Switzerland.
Dr. B. Canaud, MD, Fresenius Medical Care (FMC), Bad Homburg, Germany.