Hepatitis B virus (HBV) infection has high rates of morbidity and mortality in the general population. In chronic kidney disease (CKD), these vary depending on patient characteristics. In dialysis, its prevalence ranges from 0 to 7% to 10–20 %.1 According to the PIBHE study, its prevalence in Spain is 1.03%.2 Vaccination and use of universal protective measures have contributed to its decline.1–4 In kidney transplantation (KT), its prevalence increases as a result of risk of reactivation due to immunosuppressants.1,5–7 We report a case of HBV reactivation in a patient with previously managed infection who restarted peritoneal dialysis (PD), following failed KT, in whom immunosuppression was maintained to prevent graft intolerance syndrome (GIS).
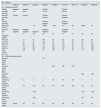
A 64-year-old man had CKD secondary to IgA glomerulonephritis. In January 2004, he presented anti-HBV markers consistent with a prior resolved infection (negative HBsAg and anti-HBc markers and positive anti-HBs markers). He had never been vaccinated against HBV. In March 2004, he started PD. Six months later, he received a KT and started immunosuppression with tacrolimus, mycophenolate mofetil (MMF) and prednisone. During the KT, MMF was replaced with everolimus. He restarted PD in July 2016 due to chronic allograft nephropathy. He had a normal liver panel and the same anti-HBV serology markers of resolved infection (Table 1). Low-dose immunosuppression with everolimus, prednisone and tacrolimus was maintained to prevent GIS. In December 2016 he was admitted due to febrile syndrome of unknown origin. It was decided to suspend everolimus and decrease the doses of prednisone and tacrolimus, with significant patient improvement. In March 2017, routine laboratory testing showed elevated transaminases. The patient was asymptomatic. An HBV DNA serology study confirmed the presence of active infection due to probable HBV reactivation (Table 1). To amplify the study, a sample was sent to the Centro Nacional de Microbiología [Spanish National Microbiology Centre (CNM)]. The results indicated: genotype H; mutation 145R in HBsAg associated with vaccine escape, resistance to immunotherapy and escape on detection of HBsAg in certain immunoassays; and two mutations in the polymerase (possible resistance) for 169 L (entecavir) and 173A (lamivudine). At first, he was treated with entecavir 0.5 mg every five days, but given his lack of response and the results from the CNM, this was replaced with tenofovir 245 mg/week. Thereafter, the patient improved considerably (Table 1). In December 2017, he was placed again on the KT waiting list. In February 2018, he underwent KT again. In May 2018, his dose of tenofovir was increased to 245 mg/24 h as he showed normal kidney function and increased HBV DNA. At present, the patient is asymptomatic and has normal laboratory and serology parameters (Table 1).
The patient's clinical, clinical chemistry and serology course.
| [0,1−10]Date | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 14/01/04 | 18/07/16 | 08/12/16 | 09/03/17 | 21/06/17 | 19/10/17 | 22/11/17 | 21/05/18 | 03/09/18 | |
| [0,1−10]Laboratory tests | |||||||||
| HBsAg | Negative | Negative | Positive | Positive | |||||
| anti-HBs (mIU/mL) | >10 | 34.34 | 313.28 | 243.62 | |||||
| anti-HBc | Positive | Positive | Positive | Positive | |||||
| anti-HBc IgM | Positive | Negative | |||||||
| HBeAg | Positive | Positive | |||||||
| anti-HBe | Negative | Negative | |||||||
| HBV DNA (IU/mL) | <10 | 40,891 | 1449 | 317 | 65 | 5890 | 97 | ||
| anti-HCV | Negative | Negative | Negative | Negative | |||||
| HIV | Negative | Negative | Negative | Negative | Negative | ||||
| P creatinine (mg/dl) | 5 | 4.6 | 4.6 | 5.4 | 4.7 | 5.1 | 0.9 | 0.9 | |
| AST (U/l) | 17 | 12 | 103 | 34 | 36 | 23 | 34 | 53 | |
| ALT (U/l) | 16 | 10 | 163 | 36 | 45 | 24 | 32 | 54 | |
| GGT (U/l) | 58 | 60 | 177 | 153 | 137 | 72 | 55 | 98 | |
| AP (U/l) | 71 | 83 | 137 | 90 | 149 | 97 | 67 | 122 | |
| LDH (U/l) | 206 | 245 | 258 | 283 | 261 | 247 | 213 | 288 | |
| CRP (mg/dl) | 0.5 | 8.1 | 1.1 | 0.8 | 0.7 | 0.7 | 1.2 | 0.6 | |
| [0,1−10] | |||||||||
| [0,1−10]Pharmacological data | |||||||||
| Entecavir doses (mg/5 days) | 0.5 | ||||||||
| Tenofovir doses (mg/7 days) | 245 | 245 | 245 | ||||||
| Tenofovir doses (mg/24 h) | 245 | 245 | |||||||
| Tacrolimus doses (mg/24 h) | 4 | 4 | 3 | 2.5 | 2.5 | 2.5 | 2 | 2 | |
| Tacrolimus levels (ng/mL) | 5.3 | 10.2 | 4.2 | 3.5 | 3.9 | 5.8 | 4.2 | 3.2 | |
| Everolimus doses (mg/24 h) | 1.25a | 1.75 | 1.25 | ||||||
| Everolimus levels (ng/mL) | 4.98 | 5.8 | 4.4 | ||||||
| Kidney status | ACKD | PD | PD | PD | PD | PD | PD | KT | KT |
ACKD: advanced chronic kidney disease; ALT: alanine aminotransferase; AP: alkaline phosphatase; AST: aspartate aminotransferase; CRP: C-reactive protein; GGT: gamma-glutamyl transferase; KT: kidney transplantation; LDH: lactate dehydrogenase; P creatinine: plasma creatinine; PD: peritoneal dialysis.
HBV reactivation in the kidney patient population has higher rates of morbidity and mortality than in the general population.1,5,6 Several authors have warned of HBV reactivations in patients on dialysis or following immunosuppressive therapy in KT.1 Risk factors (RFs) such as advanced age, graft rejection, use of rituximab and/or everolimus and loss or absence of anti-HBs markers have been reported.1,2,5,7 Our patient did not present any of these; therefore, reactivation was unlikely, but did happen. Clinical guidelines recommend annual serology monitoring for patients who test positive for anti-HBc markers, regardless of whether they test positive or negative for anti-HBs markers.1,3 Some authors recommend testing for HBV DNA in cases of negative HBsAg markers and positive anti-HBc markers to detect potential occult HBV infection.1,6 In our case, all this testing was performed, but it was a routine liver panel that alerted us. If an anti-HBc‒positive patient presents liver abnormalities, full HBV and HBV DNA serology should be performed. Early diagnosis enabled us to start immediate treatment as well as isolate and protect healthcare workers and patients on dialysis. Some authors advise starting PD in HBsAg-positive kidney patients and patients with a history of resolved HBV infection (negative HBsAg, positive anti-HBc), clinical conditions permitting.4 In our case, the patient freely chose PD. This facilitated the non-transmission of the infection. A debate on prioritising a patient's free choice of dialysis versus the safety of other patients on dialysis may be warranted. Regarding drugs, at present, entecavir and tenofovir are considered first-line options.1,6 Early molecular study enabled detection of drug resistances and an unusual genotype in Spain (genotype H, common in South America8). This allowed the patient to follow a favourable clinical course and be put back on a KT list.
Finally, patients with CKD and resolved HBV infection represent a challenge for the nephrologist. HBV reactivation may happen at any time. Liver panel monitoring may be a good method for early detection. Provided there is no medical contraindication, anti-HBc‒positive patients should start PD or home haemodialysis to prevent massive spread of infection. Molecular study of HBV is very useful for optimising treatment.
Please cite this article as: García LC, Alegre PT, Villalta Robles VM, Calvo AA, Gómez MAR, Benito MH, et al. Reactivación silenciosa del virus de la hepatitis b en paciente que reinicia diálisis tras trasplante renal ¿Cómo podemos prevenirlo o anticiparlo en el diagnóstico? Nefrologia. 2020;40:477–479.