Fabry disease may be treated by enzyme replacement therapy (ERT), but the impact of chronic kidney disease (CKD) on the response to therapy remains unclear. The aim of the present study was to analyse the incidence and predictors of clinical events in patients on ERT.
Study designMulticentre retrospective observational analysis of patients diagnosed and treated with ERT for Fabry disease. The primary outcome was the first renal, neurological or cardiological events or death during a follow-up of 60 months (24–120).
ResultsIn 69 patients (42 males, 27 females, mean age 44.6±13.7 years), at the end of follow-up, eGFR and the left ventricular septum thickness remained stable and the urinary albumin: creatinine ratio tended to decrease, but this decrease only approached significance in patients on agalsidase-beta (242–128mg/g (p=0.05). At the end of follow-up, 21 (30%) patients had suffered an incident clinical event: 6 renal, 2 neurological and 13 cardiological (including 3 deaths). Events were more frequent in patients with baseline eGFR≤60ml/min/1.73m2 (log Rank 12.423, p=0.001), and this remained significant even after excluding incident renal events (log Rank 4.086, p=0.043) and in males and in females. Lower baseline eGFR was associated with a 3- to 7-fold increase the risk of clinical events in different Cox models.
ConclusionsGFR at the initiation of ERT is the main predictor of clinical events, both in males and in females, suggesting that start of ERT prior to the development of CKD is associated with better outcomes.
El objetivo de este estudio es realizar un mapa del tratamiento actual de la enfermedad de Fabry en España, analizando el efecto de diferentes factores en el desarrollo de eventos clínicos a largo plazo.
Diseño del estudioAnálisis observacional retrospectivo multicéntrico. Criterios de inclusión: pacientes diagnosticados y tratados de enfermedad de Fabry. Se recogieron datos generales en relación con el diagnóstico, síntomas y tipo mutación, tipo de tratamiento recibido, evolución renal y cardiológica. Durante un tiempo de seguimiento de 60 meses (24-120), se recogió el primer evento clínico tras el inicio de tratamiento sustitutivo enzimático definido como mortalidad, evento renal, cardiológico o neurológico.
ResultadosSe incluyeron 69 pacientes (42 H, 27 M) con una edad media de 44,6±13,7 años. A los cinco años de tratamiento, el FGe y la hipertrofia ventricular izquierda se mantuvieron estables, y la albuminuria tiende a disminuir, siendo este descenso más significativo en el grupo de pacientes tratados con beta-galactosidasa (de 242 a 128mg/g (p=0,05). Veintiún pacientes sufrieron un evento clínico (30%): seis renales, dos neurológicos y 13 cardiológicos (incluidas tres muertes). Los pacientes con ERC (FGe<60) antes del inicio de tratamiento tuvieron más eventos (log-rank 12.423, p=0,001), manteniéndose la predicción si excluíamos los eventos renales (log-rank 4.086 (p=0,043) en hombres y mujeres. La peor función renal al inicio del tratamiento aumentó entre tres y siete veces el riesgo de eventos clínicos en diferentes modelos de Cox ajustados.
ConclusionesLa función renal al inicio de tratamiento sustitutivo enzimático es la principal predictora de desarrollo de eventos clínicos a largo plazo, tanto en hombres como mujeres. El inicio de tratamiento sustitutivo enzimático precoz antes del desarrollo de ERC mejoraría el pronóstico.
(FD) is a rare, X-linked lysosomal storage disease caused by a deficiency of alpha galactosidase A enzyme activity due to mutations in the GLA gene. Accumulation of the glycosphingolipid globotriaosylceramide (GL-3) and its derivative globotriaosylsphingosine (lyso-Gb3) causes multisystemic disease, life-threatening complications, and a reduced life expectancy in both males and females.1 GL-3 is predominantly intracellularly and lyso-Gb3 is predominantly in plasma. Target organ manifestations include progressive renal failure, cardiomyopathy and recurrent strokes/transient ischaemic attacks (TIAs), resulting in an increased risk for early death if untreated.2,3 Enzyme replacement therapy (ERT) with agalsidase (human recombinant alpha galactosidase A) reduces GL-3 inclusions and lyso-Gb3 levels.4,5 Two agalsidase preparations were approved in Europe in 2001, both administered intravenously every other week (EOW): agalsidase-alfa (Replagal®) administered at the licensed dose of 0.2mg/kg; and agalsidase-beta (Fabrazyme®) administered at the licensed dose of 1mg/kg body weight.6,7 Although ERT has been in clinical use since 2001, many questions remain regarding timing if treatment initiation, optimal dose, and treatment goals.8,9 Thus, although ERT was shown to slow disease progression,6 recent long-term analyses of agalsidase-alfa as well as agalsidase-beta treatment outcomes suggested substantially diverse frequencies of clinically relevant events while on therapy.3,10–12 A target organ affected is usually the kidney. In children, podocyte glycolipid deposits are associated with podocyte injury (foot process effacement), which precedes pathological albuminuria, which is usually the first evidence of Fabry nephropathy.13,14 This is followed by a progressive decrease in glomerular filtration rate (GFR), leading to the need for renal replacement therapy (RRT) at a mean age of 40 years in classical FD, that is, 5–15 years before the first cardiac event.15,16 The magnitude of proteinuria is a key determinant of nephropathy progression in treated and non-treated patients.12,17
In the present study we analysed the incidence of clinically relevant renal, cardialogical and neurological FD-related events in 69 patients with FD treated at Spanish centres and explored the factors predicting outcomes.
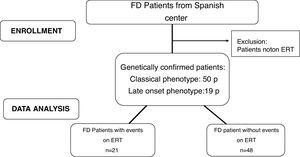
Materials and methodsStudy designMulticentre retrospective observational analysis. The study was approved by the local Ethics Committees in accordance with the Declaration of Helsinki. A flow chart of patients included in this study is showed in Fig. 1.
MethodsInclusion criteria: FD patients ≥16 years old with a classical or later-onset clinical phenotype who initiated ERT (agalsidase-alfa at doses of 0.2mg/kg or agalsidase-beta at doses of 1mg/kg each 14 days). Exclusion criteria: FD diagnosed patients who had not received ERT. The patients were sorted according to their phenotype: classic or later-onset. The classic phenotype is more severe due to very low or absent alpha-galactosidase A activity, with the typical early symptoms such as acroparesthesias, angiokeratoma, corneal opacities and hypohidrosis, particularly in males. With advancing age, the progression deposition of gycosphingolipids lead to cardiomyopathy, deterioration of kidney function or premature stroke. The later-onset phenotype is typically less severe with a significant residual enzyme activity in males, who usually lack the early symptoms but present with a cardiomyopathy or chronic kidney disease in adult age. The phenotype was classified based on the genotype according International Fabry Disease Genotype-Phenotype database.
General data were collected regarding the diagnosis, symptoms and mutation type, treatment received and clinical events: renal, neurological and cardiological. The routine assessment included renal, cardiac and neurological parameters. Renal function and injury were quantified by the estimated GFR (eGFR) based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for serum creatinine18 and by the albumin: creatinine ratio (ACR) from spot urine, respectively. Albuminuria was defined as an ACR>30mg albumin/g creatinine and CKD as eGFR<60ml/min/1.73m2.18 Cardiac assessment included echocardiography and electrocardiography. Left ventricular hypertrophy (LVH) was defined as an interventricular septum thickness in diastole (IVSD)>12mm. Neurological assessment included a clinical interview focusing on a history of stroke or TIA.
OutcomesPrimary outcome was defined as the first incident clinical event after initiation of ERT. Clinical events were defined as follows: (1) cardiac events: heart failure hospitalisation, atrial fibrillation, cardiac arrhythmia requiring an implantable cardioverter defibrillator, myocardial infarction, coronary artery bypass grafting or percutaneous transluminal coronary angioplasty, (2) renal events: progression of renal disease to CKD stage 5 [eGFR<15ml/min/1.73m2], kidney transplantation or dialysis and/or doubling serum creatinine and (3) neurological events: new stroke/TIA. Death was also recorded.
Secondary outcomes included changes in organ function and/or structure for the heart (IVSD) and for the kidney (annual eGFR and ACR).
Statistical analysisContinuous variables are expressed as mean with standard deviation (SD) or as median (interquartile range) when values were unequally distributed. Differences between groups were analysed with unpaired Student's t or Mann–Whitney U test for continuous data and Fisher's exact test for categorical data. ANOVA was used to determine the influence of two variables (such us time and gender or time and type of ERT) on renal and cardiological parameters. The Kaplan–Meier method was used for survival plots. Cox proportional hazard models were used to determine the influence of different variables before ERT initiation on outcomes adjusted for age and gender. All statistical analyses were performed with SPSS 20.0 software (SPSS; Chicago, IL, USA). Statistical significance was considered as a two-sided p-value <0.05.
ResultsIn this retrospective multicentre longitudinal study, a total of 69 (27 females, mean age at diagnosis 42±15 years) genetically confirmed patients with a clinical classical or later-onset phenotype of FD from centres in Spain were analysed. We assessed the incidence of first renal, cardiac, or neurological event or death during long-term ERT (median time on ERT 60 (24–120) months). Table 1 provides a comprehensive overview of the baseline characteristics of the study group divided by gender. Males were younger (p=0.006) and had lower eGFR (p=0.004) at the time of ERT initiation but did not display higher IVSD and males more frequently carried later-onset phenotype (p=0.018) (Table 1).
Baseline characteristics of FD patients divided according sex.
| Males (n=42) | Females (n=27) | p | |
|---|---|---|---|
| Age at diagnosis (years) | 38.9±13.7 | 46.6±16.5 | 0.040 |
| Age at baseline (years) | 41.0±12.3 | 50.1±14.3 | 0.006 |
| eGFR at baseline (ml/min/1.73m2) | 61.2±36.1 | 84.4±27.1 | 0.004 |
| ACR at baseline (mg/g) | 242 (65–1200) | 123 (10–786) | 0.163 |
| SBP at baseline (mmHg) | 127±20 | 120±16 | 0.192 |
| DBP at baseline (mmHg) | 75±15 | 74±13 | 0.844 |
| IVSD at baseline(mm) | 14.4±4.4 | 14.1±5.9 | 0.795 |
| RAAS blockers (%) | 78% | 59% | 0.290 |
| Anhidrosis | 33% | 33% | 1.000 |
| Angiokeratoma | 40% | 37% | 0.624 |
| Acroparesthesia | 40% | 41% | 1.000 |
| Symptoms at diagnosis | |||
| Renal | 11 | 3 | |
| Cardiological | 5 | 4 | |
| Neurological | 2 | 5 | |
| Systemic symptoms | 20 | 11 | |
| Asymptomatic | 4 | 4 | |
| Classic/later-onset phenotype | 24/18 | 23/4 | 0.018 |
| ERT (agalsidase alfa/beta) | 18/24 | 14/13 | 0.629 |
| Renal, cardiological and neurological events prior to ERT (%) | 33% | 33% | 1.000 |
eGFR: estimated glomerular filtration rate (calculated used CKD-EPI formula, IVSD: interventricular septum thickness in diastole (mm). RAAS: renin–angiotensin–aldosterone system. Symptoms at diagnosis: renal (urinary alterations, chronic kidney disease, albuminuria, dialysis or transplantation), cardiological (cardiac arrythmia, myocardial infarction, coronary artery bypass or angioplasty), neurological(stroke/TIA or acroparesthesia), systemic symptoms (combination of other symptoms, gastrointestinal symptoms, angiokeratomas or anhidrosis).
An overview of GLA mutations and their clinical phenotypes is provided in Table 2. No differences were found among baseline characteristics of FD patients divided according to ERT type (data are not showed).
Overview of mutations of GLA and their phenotypes. Note: in 5 patients the mutation has not been collected and in 1 patient the mutation has not been identified. The phenotype was classified based on the genotype according International Fabry Disease Genotype-Phenotype database.
| Nomenclature | Amino acid change | Nucleotide change | Exon involved | Mutation type | Phenotype | Number of patients |
|---|---|---|---|---|---|---|
| S238N | p.Ser238Asn | c.713G>A | Exon 5 | Missense | Later-onset | 10 |
| W47G | p.Trp47Gly | c.139T>G | Exon 1 | Missense | Classic | 6 |
| A368Lfs*25. | p.A368delinsFYfs 23 | c.1102delGinsTTATAC | Exon 7 | Missense | Classic | 3 |
| D92G | p.Asp92Gly | c.275A>G | Exon 2 | Missense | Classic | 3 |
| G183V | p.Gly183Val | c.548G>T | Exon 4 | Missense | Classic | 3 |
| A368T | p.Ala368Thr | c.1102G>A | Exon 7 | Missense | Benign | 2 |
| I91T | P.Ile91Thr | c.272T>C | Exon 2 | Missense | Classic | 2 |
| R227Q | p.Arg227Gln | c.680G>A | Exon 5 | Missense | Classic | 2 |
| F113L | p.Phe113Leu | c.337T>C | Exon 2 | Missense | Later-onset | 2 |
| A143P | p.Ala143Pro | c.427G>C | Exon 3 | Missense | Classic | 2 |
| R342L | p.Arg342Leu | c.1025G>T | Exon 7 | Missense | Classic | 1 |
| R220* | p.Arg220Ter | c.658C>T | Exon 5 | Nonsense | Classic | 1 |
| Q119* | p.Gln119Ter | c.355C>T | Exon 2 | Nonsense | Classic | 1 |
| R220Q | p.Arg220Gln | c.659G>A | Exon 5 | Missense | Likely benign | 1 |
| K185E | p.Lys185Glu | c.553A>G | Exon 4 | Missense | Later-onset | 1 |
| I407K | p.Ile407Lys | c.1220T>A | Exon 7 | Missense | Classic | 1 |
| 370_639del | p.Val124_Lys213del | 370_639del | Exon 3 and 4 | Deletion | Classic | 1 |
| Q386P | p.Gln386Pro | c.1157A>C | Exon 7 | Missense | Classic | 1 |
| R301* | p.Arg301Ter | c.901C>T | Exon 6 | Nonsense | Classic | 1 |
| R112C | p.Arg112Cys | c.334C>T | Exon 2 | Missense | Classic | 1 |
| A73E | p.Ala73Glu | c.218 C>A | Exon 2 | Missense | Later-onset | 1 |
| M1T | p.Met1Thr | c.2T>C | Exon 1 | Missense | Classic | 1 |
| G361del | p.Gly361del | c.11080_1082del TGG | Exon 7 | Missense | Classic | 1 |
| G373D | p.Gly373Asp | c.1118G>A | Exon 7 | Missense | Classic | 1 |
| D170V | p.Asp170Val | c.509A>T | Exon 3 | Missense | Classic | 1 |
| D313Y | p.Asp313Tyr | c.937G>T | Exon 6 | Missense | Benign | 1 |
| V124D | p.Val124Asp | c.371T>A | Exon 3 | Missense | Classical | 1 |
| C378Y | p.Cys378Tyr | c.1133G>A | Exon 7 | Missense | Classic | 1 |
| Y200C | p.Tyr200Cys | c.599A>G | Exon 4 | Missense | Classic | 1 |
| R118C | p.Arg118Cys | c.352C>T | Exon 2 | Missense | Benign | 1 |
| I270T | p.Ile270Thr | c.809T>C | Exon 6 | Missense | Classic | 1 |
| Q279R | p.Gln279Arg | c.836A>G | Exon 6 | Missense | Classic | 1 |
| G411D | p.Gly411Asp | c.1232G>A | Exon 7 | Missense | Classic | 1 |
| G260R | p.Gly260Arg | c.778G>A | Exon 5 | Missense | Classic | 1 |
| D155H | p.Asp155His | c.463G>C | Exon 3 | Missense | Classic | 1 |
| L191P | p.Leu191Pro | c.572T>C | Exon 4 | Missense | Classic | 1 |
| L206P | p.Leu206Pro | c.617T>C | Exon 4 | Missense | Classic | 1 |
| M208Yfs*24. | p.Met208TyrfsTer24 | c.621dupT | Exon 4 | Small insertion | Classic | 1 |
After of a median of 5 years on ERT, eGFR changed from 61.2±36.1 to 64.9±33.1ml/min/1.73m2 in males and from 84.4±27.1 to 92.1±39.4ml/min/1.73m2 in females (p=0.004 between gender) and albuminuria changed from 242 (65–1200) to 540 (40–958)mg/g in males and from 123 (10–786) to 86 (21–176) in females. Higher percentage of males were on renin–angiotensin–aldosteron system inhibitors (RAASi) at the beginning of ERT, but differences with females was not statistically significant. IVSD also was not modified in males nor in females after initiation of ERT (14.4±4.4 to 14.7±2.6 in males and 14.1±5.9 to 14.1±4.6 in females).
Evolution of renal and cardiological parameters according to ERT typeAfter 5 years on ERT, eGFR remained stable in patients treated with agalsidase-alfa or agalsidas-beta (eGFR change from 76 to 77 in alfa, from 65 to 68ml/min/1.73m2 in beta), while albuminuria tended to decrease in patients treated with agalsidase-beta (242–128mg/g (p=0.052), and the left ventricular hypertrophy was not modified.
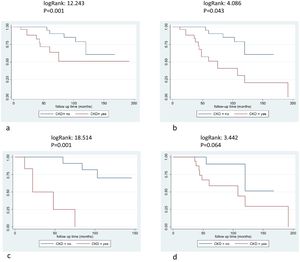
OutcomesDuring ERT, 21 patients (30%) suffered a first incident clinical event: 13 males (31%) and 8 females (30%). The distribution of clinical events was: six renal, 13 cardiac (3 cardiovascular deaths included) and 2 neurological events. New cardiac events included four cases of heart failure, four cases of arrhythmia/atrial fibrillation, two implantable cardioverter device/pacemaker implantations and three myocardial infarctions. Neurological events included two strokes and renal events included patients starting renal replacement therapy (5 dialysis and 1 renal transplant). Patients suffering from an event while on ERT presented a significantly lower eGFR at baseline compared with those without events (p=0.014) and higher ACR values (p=0.043) (Table 3). Most patients who suffered a clinical event after ERT carried mutations related with likely classic phenotype (19 out of 21 patients) (p=0.011). The age at the start of ERT, gender or previous clinical events were not different between patients who suffered or not incident clinical events (Table 3). Patients with CKD before the start of ERT had more frequent events (log Rank 12.423, p=0.001), even after excluding incident renal events (log Rank 4.086, p=0.043) and these differences were more relevant in females (log Rank 18.514, p<0.001 for all events and females and logRank 3.442, p=0.064 for males) (Fig. 2a–d). Baseline CKD predicted the incidence of clinical events in different Cox models, adjusted for gender, treatment type, age at start of treatment, phenotype, and RAASi treatment (Table 4).
Differences between patients who suffered or not incident.
| Clinical events (n=21) | No clinical events (n=48) | p | |
|---|---|---|---|
| Sex | 13 males, 8 females | 29 males, 19 females | 1.000 |
| eGFR (ml/min/1.73m2) | 55.1±31.9 | 77.2±33.8 | 0.014 |
| ACR(mg/g) | 440(47–1839) | 115(34–629) | 0.043 |
| IVSD(mm) | 15.6±4.7 | 13.7±5.0 | 0.167 |
| Age at start of ERT | 48.1±11.2 | 43.0±14.6 | 0.162 |
| Phenotype (classic/later-onset) | 19/2 | 28/20 | 0.011 |
| Previous clinical events (%) | 38% | 31% | 0.591 |
Survival free clinical events according chronic kidney disease. 2a: all population and clinical events, logRank: 12.423, p=0.001, 2b: non-renal events, logRank: 4.086, p=0.043, 2c: female patients and all clinical events, logRank: 18.514, p=0.001, 2d: male patients and all clinical events, logRank: 3.442, p=0.064.
Factors influencing incidence of clinical events after ERT. Cox regression analysis.
| Variables | HR (95% IC) | p |
|---|---|---|
| Baseline age (years) | 1.041 (1.003–1.080) | 0.034 |
| Gender (female) | 1.060 (0.432–2.603) | 0.898 |
| Phenotype(later-onset) | 0.294 (0.068–1.270) | 0.101 |
| Treatment type (agalsidase beta) | 1.672 (0.690–4.056) | 0.255 |
| ACR (mg/g) | 1.001 (1.000–1.001) | 0.026 |
| CKD G3–G5 (yes) | 4.568 (1.794–11.733) | 0.001 |
| RAASi (yes) | 2.012 (0.672–6.023) | 0.212 |
| Pre-ERT clinical events (yes) | 1.215 (0.483–3.057) | 0.679 |
| CKD vs age | 4.205 (1.641–10.775) | 0.003 |
| CKD vs gender (male) | 7.214 ((2.393–21.752) | 0.001 |
| CKD vs ERT (agalsidase-beta) | 4.549 (1.772–11.678) | 0.002 |
| CKD vs RAASi | 4.323 (1.668–11.203) | 0.003 |
| CKD vs phenotype(later-onset) | 3.979 (1.545–10.248) | 0.004 |
| CKD vs ACR | 2.816 (0.992–7.994 | 0.052 |
ACR: albumin/creatine ratio, CKD G3-G5: chronic kidney disease with eGFR<60ml/min/1.73m2. RAASi: renin–angiotensin–aldosterone system inhibitors.
In this study, we analysed the incidence of clinical events in patients with FD on long-term ERT and identified differences between patients suffering or not suffering events. Our main finding was the negative impact of baseline renal function at the start of ERT on incident clinical events in a population with lower baseline eGFR and higher use of RAS blockers than prior reports. CKD at the start of ERT increased 3- to 7-fold the risk of clinical events while on therapy. Another key and novel finding is that the impact of CKD on outcomes was also present, and even to a higher extent, in females as compared to males.
Thirty per cent of our patients on ERT during a median of 60 months developed a clinically relevant event. This percentage is slightly higher than the incidence of clinical events reported for patients on agalsidase-beta (17%–19%),19,20 but lower than published for agalsidase-alfa (50%).21,22 Our patient characteristics differ from these prior reports mainly in the lower eGFR at the start of ERT, both in men and in women (61±36 and 84±27ml/min/1.73m2, respectively), probably as patients were treated mostly in nephrology units. In this regard, and again different from some prior reports, a high percentage of patients were on RAS blockers. In prior reports, age, gender and classic phenotype were strong predictors of the risk to develop clinical events while on ERT.10,11,20,23–26 An insufficient sample size or a higher prevalence of lower eGFR may have precluded to reproduce some of these findings. Thus, only age, baseline renal function and albuminuria were predictors of incident clinical events, and not only of renal events, but also of cardiological and neurological events.
The present results agree with some prior reports but also add novel findings. Thus, Lenders et al.21 reported that in a cohort of 54 FD patients (28 males) on ERT for 81±21 months, a decreased baseline eGFR (<75ml/min/1.73m2) was associated with an increased risk for cardiovascular [HR 3.59 (95% CI 1.15–11.18); p=0.0273] or combined renal, cardiac and neurological end points [HR 4.77 (95% CI 1.93–11.81); p=0.0007] while on ERT. However, no results were reported independently for males or females and their patients had less severe kidney disease (higher baseline eGFR at 87ml/min/1.73m2 and lower baseline ACR) and lower use of RAS blockers. In a recently published retrospective study of 293 FD patients on ERT by Arends et al.,27 while age, gender and phenotype were predictors of event rates, eGFR was the most important predictor. Thus, the hazard ratios increased from 2 at eGFR<90ml/min/1.73m2 to 4 at eGFR<30, compared to patients with an eGFR>90ml/min/1.73m2. These results are like ours, however in Arends’ study, the influence of baseline renal function was only significant in classical male patients. Thus, for the first time, we show that initiation of ERT in women before renal function deteriorates has a similar or even larger impact as in Fabry males to prevent clinical events.
No significant changes were observed in renal function and cardiac parameters (eGFR or IVSD) during the observation period, while trends to improvement were observed for ACR. In addition, we did not observe an influence of the type of treatment or gender on changes in these parameters. In the literature, some studies show no change in kidney or cardiac parameters over time while on ERT.22 Since Fabry disease is a lifelong progressive debilitating disease associated with premature mortality, no change in these variables is an indication of disease stabilisation.
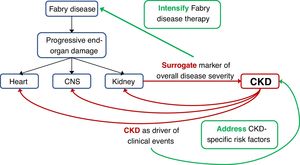
An issue not addressed in the present report is whether the increased risk of clinical events is direct consequence of more advanced Fabry disease in the different target organs, being the severity of kidney disease a surrogate marker of overall FD severity, versus an alternative explanation in which the increased risk of clinical events would be related to CKD itself. This is not a mere theoretical exercise: if it represents advanced FD, patients with FD-related CKD might benefit from aggressive initial therapy for FD, perhaps even involving the simultaneous initial use of two different FD treatments (e.g. ERT and chaperone or ERT and substrate reduction therapy when this becomes available). However, CKD itself is associated with an increased risk of CKD progression, cardiac events and stroke.28 If this were the main driver of the increased incidence of clinical events, then aggressive therapy of CKD-related risk factors by nephrologists may improve outcomes. In favour of this later hypothesis, a low GFR was an independent predictor of events when adjusted for age, which may be considered another surrogate for overall severity of end-organ damage.
Our study has several limitations. First, it is a retrospective and multicentric study which can influence the data collection. Second, the median follow-up was relatively short (5 years) for a disease that have a natural history of 40 years up to the first severe clinical event. In this regard, the Canadian Fabry Disease Initiative Trial required a follow-up of 10 years to demonstrate statistically significant difference for the renal events.3 Third, the sample size is relatively small, although larger than other observational studies independent from industry and lyso-Gb3 levels were not available. By last, recent studies demonstrated the clinical impact of neutralizing anti-drug antibodies in male patients with Fabry disease,29 but in our study, blood samples were not available to determine antibody titers. This fact could be a bias about the effect of agalsidase-alfa or beta in the outcomes.
In conclusion, renal function at the start of ERT is the main predictor of the development of long-term clinical events even when differences related to type of treatment or type of mutation were not observed. As a novel finding, this is also the case in female patients, suggesting that ERT should be initiated early in the course of the disease, early meaning according to the present report, at least before renal function decreases. Further studies should address whether more aggressive therapy of FD and/or CKD-related risk factors improves outcomes (Fig. 3).
Conclusions1. Baseline renal function is the main predictor of the long-term clinical events.
2. Enzyme replacement therapy should initiate early before renal function decreases.
3. Renal function predicts outcomes in males and females.
FundingMG, JT, AO, RT are supported by ISCIII RETIC REDINREN, RD016/009 and FEDER funds.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestMG has received research support from Sanofi Genzyme and has received speaker honoraria and travel support from Sanofi Genzyme and Shire. MLM, AO, RT are consultants for Amicus Therapeutics, Sanofi Genzyme and Shire; have received research support from Sanofi Genzyme and Shire and have received speaker honoraria and travel support from Amicus Therapeutics, Sanofi Genzyme, and Shire. RMA has received speaker honoraria from Shire and travel support from Sanofi Genzyme. FD has received travel grants and speaker fees from Takeda and Amicus. IM received speaker honoraria and travel support from Sanofi Genzyme and Shire. IA has received travel and courses supported from Shire.