The study aimed to investigate the role of magnesium sulfate prophylaxis in nephrotoxicity caused by colistin. Thirty Wistar Albino rats were divided into four groups: control, colistin, magnesium (Mg), and Mg+colistin. The drugs were administered to the groups for seven days. Urea-creatinine values were measured at the beginning (T0) and end (T1) of the study. Malondialdehyde (MDA) levels were measured in plasma and kidney tissue, glutathione (GSH) levels were analyzed in the erythrocyte and kidney tissues. At the end of the study, the semiquantitative score (SQS) was calculated by the histopathological examination of the kidneys. Urea values significantly decreased in Mg and Mg+colistin groups compared to the baseline (p=0.013 and p=0.001). At the time of T1, these groups had significantly lower urea values than the colistin and control groups. Creatinine value was significantly increased in the colistin group compared to baseline (p=0.005), the creatinine value in the colistin group was significantly higher than the Mg+colistin group (p=0.011). Plasma MDA levels were significantly higher in the colistin group compared to the other groups at the time of T1 (p<0.001). The Mg+colistin group had lower renal MDA levels than the colistin group. The colistin group had significantly higher renal tubular grade (p=0.035), renal affected area (p<0.001), and SQS (p=0.001) than the Mg+colistin group. The results of the study suggested that Mg sulfate may have a nephrotoxicity-reducing effect on colistin.
El objetivo del estudio fue investigar la función de la profilaxis con sulfato de magnesio en la nefrotoxicidad causada por la colistina. Se dividieron 30 ratas Wistar albinas en 4 grupos: control, colistina, magnesio (Mg) y Mg + colistina. Los fármacos se administraron a los grupos durante 7 días. Los valores de urea-creatinina se midieron al principio (T0) y al final (T1) del estudio. Se midieron los niveles de malondialdehído (MDA) en el plasma y el tejido renal, y se analizaron los niveles de glutatión (GSH) en los eritrocitos y el tejido renal. Al final del estudio, se calculó la puntuación semicuantitativa (semiquantitative score [SQS]) mediante el examen histopatológico de los riñones. Los valores de urea disminuyeron significativamente en los grupos de Mg y Mg + colistina en comparación con los valores iniciales (p = 0,013 y p = 0,001). En el momento del T1, estos grupos tenían valores de urea significativamente más bajos que los grupos de colistina y de control. El valor de creatinina se incrementó significativamente en el grupo de colistina en comparación con el valor inicial (p = 0,005); el valor de creatinina en el grupo de colistina fue significativamente mayor que en el grupo de Mg + colistina (p = 0,011). Los niveles de MDA en el plasma fueron significativamente más altos en el grupo de colistina en comparación con los otros grupos en el momento del T1 (p < 0,001). El grupo de Mg + colistina presentó niveles renales de MDA más bajos que el grupo de colistina. El grupo de colistina presentó un grado tubular renal (p = 0,035), un área renal afectada (p < 0,001) y una SQS (p = 0,001) significativamente mayores que el grupo de Mg + colistina. Los resultados del estudio indicaron que el sulfato de Mg puede tener un efecto reductor de la nefrotoxicidad de la colistina.
Colistin has been out of trade since 1970, however, it is possible to see more of this drug due to the increasing gram-negative bacterial infections to multiple drug resistance.1–3 It is used in the treatment of infections caused by carbapenem-resistant Enterobacteria, Acinetobacter baumannii, Pseudomonas aeruginosa.4 Renal failure occurs in 20–60% of colistin users.5 The studies on antioxidant molecules such as hesperidin,6 black garlic extract,7 curcumin,8 N-acetylcysteine,9 ascorbic acid,10 melatonin,11 lycopene,12 luteolin,13 grape seed extract14 found them to be effective in the prevention of colistin-induced nephropathy.
Magnesium, an antioxidant macromineral, also has the advantages of being low in cost and easy to obtain. Various agents such as contrast agents and chemotrophic agents reduce nephrotoxicity with these antioxidant effects.15–18
GSH levels, an endogenous antioxidant molecule, are decreased with colistin treatment8,12 and increased levels of MDA; which are indicative of lipid peroxidation and increased in the case of oxidative damage.19 Therefore, we examined whether magnesium had an antioxidant effect by analyzing different analytes such as GSH and MDA. The effects of colistin on the kidney have been evaluated histopathologically in some studies. The SQS system, which is the sum of tubular damage in renal tissues and the percentage of tissue damage, was used in these studies.9,13 We also planned to evaluate the colistin-induced renal tissue damage rate using this scoring system.
This study aimed to determine whether magnesium decreases nephrotoxicity caused by colistin, which found to have an antioxidant effect in various drug studies of contrast and chemotherapeutic agents.
MethodsAnimalsThe study was conducted in Selçuk University Experimental Medicine Application and Research Center with 30 male Wistar Albino rats weighing 300–350g. The study was approved by the ethics committee of the same center (number: 2019-46). Rats were kept in cages at 21±1°C for 12h in the light and 12h in the dark and they were provided food and water. To measure basal urea and creatinine levels, 1ml of blood was taken from the tail vein under anesthesia with 35mg/kg ketamine and 5mg/kg xylazine.
DrugsColistimethate sodium was provided by Kocak Farma Pharmaceutical Company, Istanbul, Turkey (Colimycin). The drug contained 4,500,000IU=384mg colistimethate sodium and it had 150mg of colistin base activity. When it was reconstituted with 2ml injection water, a 1ml solution contained 2,250,000IU colistimethate sodium. 15% of magnesium sulfate (1500mg in 10ml, Kadıköy, Istanbul, Turkey) was used in the preparation of magnesium sulfate.
Experimental designFour groups were formed for the study:
- -
The control group (n=6) was given 2ml/kg/day saline two times a day for seven days.
- -
Colistin group (n=8) was given 300,000U/kg/day colistimetate sodium two times a day for seven days.
- -
Mg group (n=8) was given magnesium sulfate intraperitoneally at a dose of 50mg/kg/day two times a day for seven days.
- -
Mg+colistin (n=8) was given intraperitoneal Mg sulfate at a dose of 50mg/kg/day, after 30min, 300,000U/kg/day of colistimethate sodium was administered two times a day for seven days.
It could also be two doses with an interval of 8h between the administration of doses. On the seventh day, rats were anesthetized with 90mg/kg ketamine and 10mg/kg xylazine 16h after the last drug administration. Right kidneys were fixed with 10% formalin for histopathological examination and left kidneys were kept at −80°C for analysis of GSH and MDA levels at the tissue level. Rats were sacrificed after taking 2ml of intracardiac blood to analyze urea, creatinine, MDA, and GSH analysis.
Biochemical measurementsBiochemical analysis was performed by a blinded biochemist.
Blood samplesBlood samples were taken into EDTA anticoagulant tubes and centrifuged at 3000 rpm for 10 min. Plasma samples were collected for the analysis of MDA, urea, and creatinine samples were collected and washed three times with an ice-cold saline solution to obtain erythrocytes to test for GSH levels in the analysis of the red blood cells.
Tissue collection and homogenizationKidney tissues were weighed, recorded, and divided into pieces and put into tubes. Samples were placed in a Misonix Microson Ultrasonic Cell Disruptor with 150mM KCl at 4°C to obtain 10% homogenates. MDA, GSH, and protein levels were analyzed in obtained homogenates. GSH concentrations were analyzed according to the Ellmann method. All analyses were carried out using spectrophotometrical techniques.20 The values were expressed as mg/g protein in kidney tissue and mg/g hemoglobin in the erythrocyte. MDA concentrations were determined by the method of Uchiyama and Mihara with spectrophotometrical techniques.21 The values were expressed as nmol/ml and nmol/g tissue. Blood hemoglobin concentrations were determined by Drabkin's method22 and calculated as g/dl. Tissue protein concentrations were analyzed with a colorimetric test kit (BioRad, cat no: 500-0002) calculated as g/dl.
Plasma urea was analyzed using a colorimetric test kit (Elabsience Urea Kit, cat no: E-BC-K183-S). The kit's detection range was 0.114–30mmol/l, inter-assay CV is 4.7%, intra-assay CV is 4.6% and sensitivity 0.114mmol/l. Plasma creatinine levels were determined with the ELISA test kit (Elabscience rat Creatinine, cat no: E-EL-0058). The detection range was 1.25–80μg/ml, and the sensitivity of the kit was 0.75μg/ml. Plasma urea and creatinine concentrations were expressed as mg/dl. BMG LABTECH (Germany) and Rayto Microplate washer (Elisa Washer) (RT-2600, China) were used for the analysis.
Histopathological examinationThe right kidney tissues for histopathological examination were fixed after 24h fixation in 10% buffered formalin. Microtome sections were taken from paraffin-block tissues and stained with hematoxylin–eosin (HE) and histochemical periodic acid Schiff (PAS) stains. Histopathological assessment was performed by a blinded pathologist. For each kidney tissue examined at 200× magnification on light microscopic examination, it was divided into three groups based on the tubular damage (grade I, grade II, and grade III). The tissue was divided into six scores based on the percentage of damage. Percentage of affected area; <1%, 0; 1–4%, 1; 5–9%, 2; 10–19%, 3; 20–29%, 4; 30–39% 5; ≥40% were scored six. These scores and grade grades were collected and divided into six groups using the SQS system within the scope of the kidney to be affected by the drug. SQS +1: mild damage (total score 1–14), SQS +2: mild to moderate damage (total score 15–29), SQS +3: moderate damage (total score 30–44), SQS +4: moderate to severe damage (total score 45–59), SQS +5: severe damage (total score 60).9,13
Power analysisIn the present study, the sample size was calculated based on the “resource equation” proposed by Arifin and Zahiruddin. The sample size in each group should be a minimum of 10/k+1 and a maximum of 20/k+1 (k the number of groups) in this equality. Thereby, we calculated the sample size of each group to be a minimum of four, and a maximum of six.23
Data analysisData were analyzed using R Version 3.6.0 (www.r-project.org). Variables were described as mean±standard deviation or median (interquartile range). The two-way repeated-measures ANOVA was performed to assess the groups’ effects and time on urea and creatinine values in rats. The box-plot method showed that there were no extreme outliers. Shapiro–Wilk's test of normality was used to analyze the distribution of data (p>0.05). Levene's test of homogeneity of variances found variances to be homogeneous (p>0.05). A Bonferroni adjustment was used for the interaction and main effects. Because the plasma MDA was not normally distributed, Kruskal–Wallis and Wilcoxon tests were used to compare groups and time effects, respectively. After the Kruskal–Wallis test results were significant, the Conover-Iman posthoc test with Bonferroni adjustment was used for multiple comparisons. One way ANOVA and Welch tests were used to compare groups in terms of GSH and MDA levels. Tukey HSD and Games-Howell posthoc tests were used for multiple comparisons according to these parameters at T1 time. Mann–Whitney-U test was used to compare groups according to the grade of renal histology, renal affected area score, and SQS. A p-value of less than 0.05 was considered as significant.
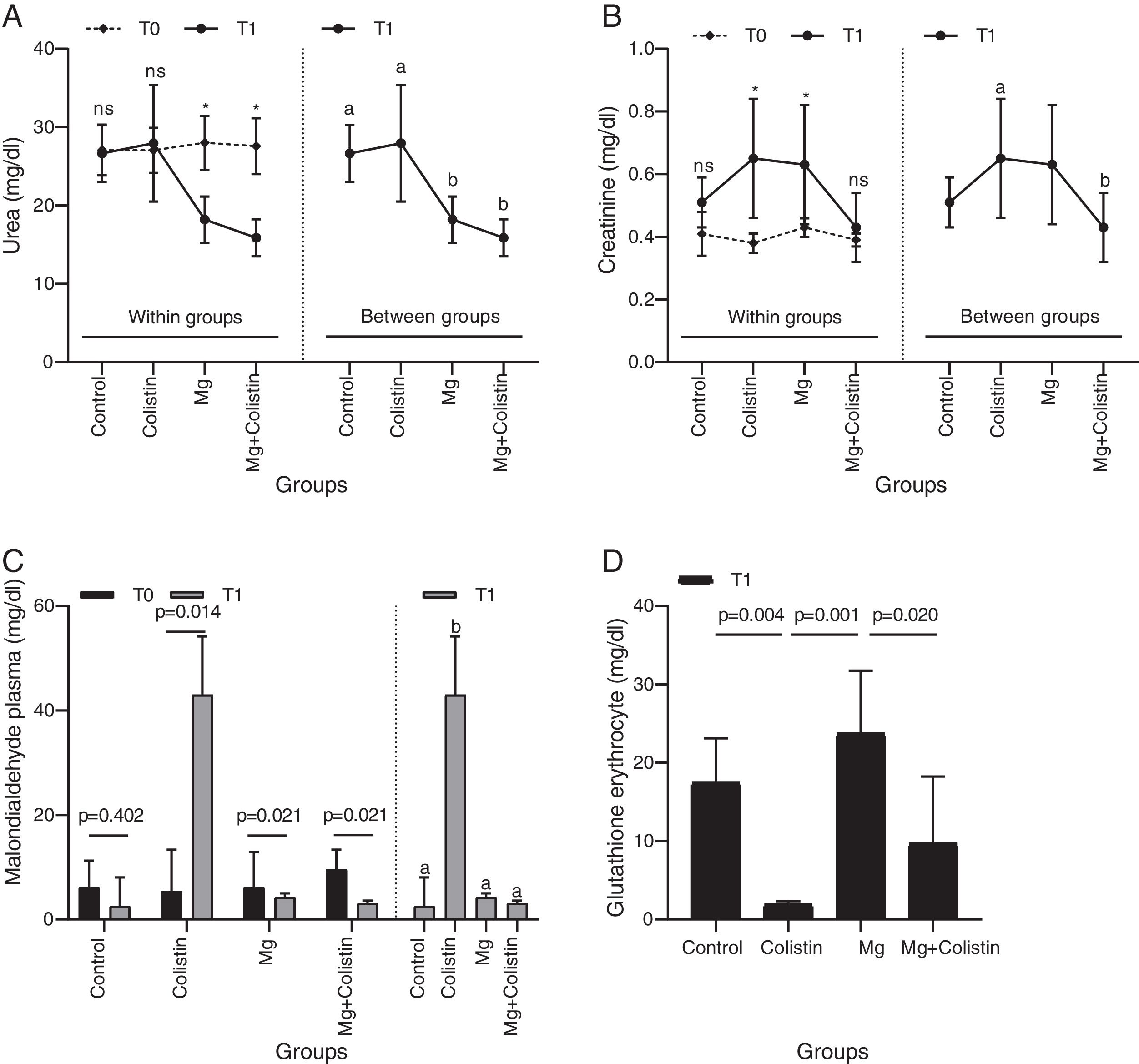
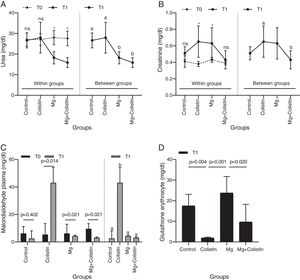
ResultsBiochemical evaluationsThe effect of different study groups overtime on urea values was assessed. There were significant main effects of group on the urea values and also there was a significant interaction between group and time on urea values in rats (F(3,26)=7.114, p=0.001, η2p=0.451). The simple main effect of the group was not significant at the time point T0 (p=0.925). It became significant at T1 (p=0.015). The mean urea value was significantly higher in the control and colistin groups compared to Mg (p=0.013 and p=0.001, respectively) and Mg+colistin (p=0.001 and p<0.001, respectively) groups at T1. Unlike the Mg (p=0.002) and Mg+colistin (p=0.001) groups, the simple main effect of time was not significant in the control (p=0.999) and colistin groups (p=0.999). The Mg (p<0.001) and Mg+colistin groups (p<0.001) had significantly lower mean urea value at the T1 time point compared to T0 (Table 1 and Fig. 1A).
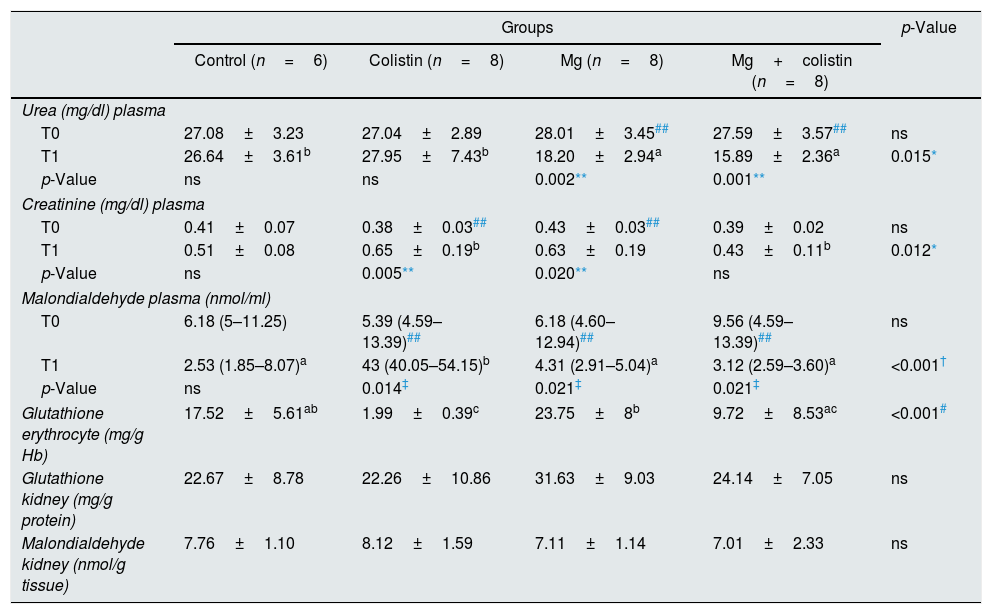
Comparison of biochemical and oxidative stress parameters.
| Groups | p-Value | ||||
|---|---|---|---|---|---|
| Control (n=6) | Colistin (n=8) | Mg (n=8) | Mg+colistin (n=8) | ||
| Urea (mg/dl) plasma | |||||
| T0 | 27.08±3.23 | 27.04±2.89 | 28.01±3.45## | 27.59±3.57## | ns |
| T1 | 26.64±3.61b | 27.95±7.43b | 18.20±2.94a | 15.89±2.36a | 0.015* |
| p-Value | ns | ns | 0.002** | 0.001** | |
| Creatinine (mg/dl) plasma | |||||
| T0 | 0.41±0.07 | 0.38±0.03## | 0.43±0.03## | 0.39±0.02 | ns |
| T1 | 0.51±0.08 | 0.65±0.19b | 0.63±0.19 | 0.43±0.11b | 0.012* |
| p-Value | ns | 0.005** | 0.020** | ns | |
| Malondialdehyde plasma (nmol/ml) | |||||
| T0 | 6.18 (5–11.25) | 5.39 (4.59–13.39)## | 6.18 (4.60–12.94)## | 9.56 (4.59–13.39)## | ns |
| T1 | 2.53 (1.85–8.07)a | 43 (40.05–54.15)b | 4.31 (2.91–5.04)a | 3.12 (2.59–3.60)a | <0.001† |
| p-Value | ns | 0.014‡ | 0.021‡ | 0.021‡ | |
| Glutathione erythrocyte (mg/g Hb) | 17.52±5.61ab | 1.99±0.39c | 23.75±8b | 9.72±8.53ac | <0.001# |
| Glutathione kidney (mg/g protein) | 22.67±8.78 | 22.26±10.86 | 31.63±9.03 | 24.14±7.05 | ns |
| Malondialdehyde kidney (nmol/g tissue) | 7.76±1.10 | 8.12±1.59 | 7.11±1.14 | 7.01±2.33 | ns |
Values are presented as mean±standard deviation or median (interquartile range), ns: not significant.
The effect of different study groups overtime on creatinine values was evaluated. There was a significant main effects of group (F(3,26)Y=Y3.294, p=0.036, η2p=0.275) and time (F(1,26)Y=Y27.334, p<0.001, η2p=0.513) on the creatinine values in rats, and also a significant interaction between group and time on creatinine values in rats (F(3,26)Y=Y3.128, p=0.043, η2p=0.265). The simple main effect of the group was not significant at the time point T0 (p=0.280). It was significant at T1 (p=0.012). The colistin group had a significantly higher mean creatinine value than the MgY+Ycolistin (p=0.011) group at T1. The simple main effect of time was not significant in the control (p=0.572) and Mg+colistin groups (p=0.999). The mean creatinine value was significantly higher at the T1 time point compared with the T0 time point for colistin and Mg groups (p=0.005 and p=0.020, respectively) (Table 1 and Fig. 1B).
MDA and GSH levelsPlasma MDA values were significantly affected by groups at the T1 time (H(3)=17.502, p<0.001), but not at T0 time (H(3)=0.121, p=0.989). The median plasma MDA value was significantly higher in the colistin group compared with the other (p<0.001) groups at the T1 time point. The median plasma MDA value was significantly higher at the T1 time point compared with the T0 time point for colistin, Mg, and Mg+colistin groups (p=0.014, p=0.021, and p=0.021, respectively) (Table 1 and Fig. 1C). There was a significant difference between the groups’ erythrocyte GSH levels (Welch F=32.715, p<0.001). The colistin group (1.99±0.39) had significantly lower GSH erythrocyte values than the control (17.52±5.61) and Mg (23.75±8) groups after treatment. There was no significant difference between the colistin and Mg+colistin (9.72±8.53) groups. Moreover, GSH erythrocyte values in rats were significantly higher after treatment in the Mg group compared to the colistin and Mg+colistin groups. There was no significant difference between the Mg and control groups (Table 1 and Fig. 1D). There were no significant differences between the groups’ (F=1.816, p=0.169, F=0.802, p=0.504, respectively) kidney GSH and MDA levels (Table 1).
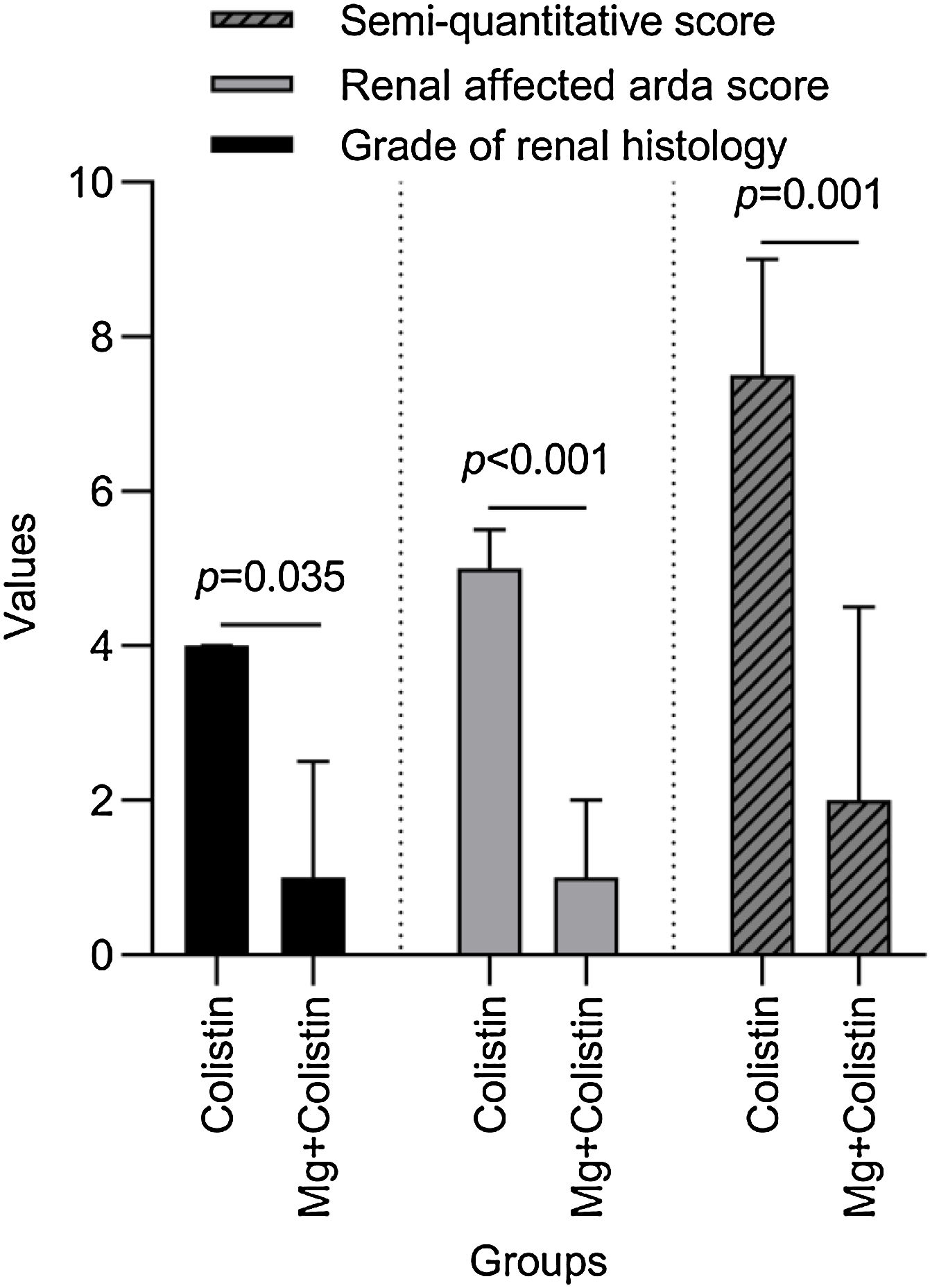
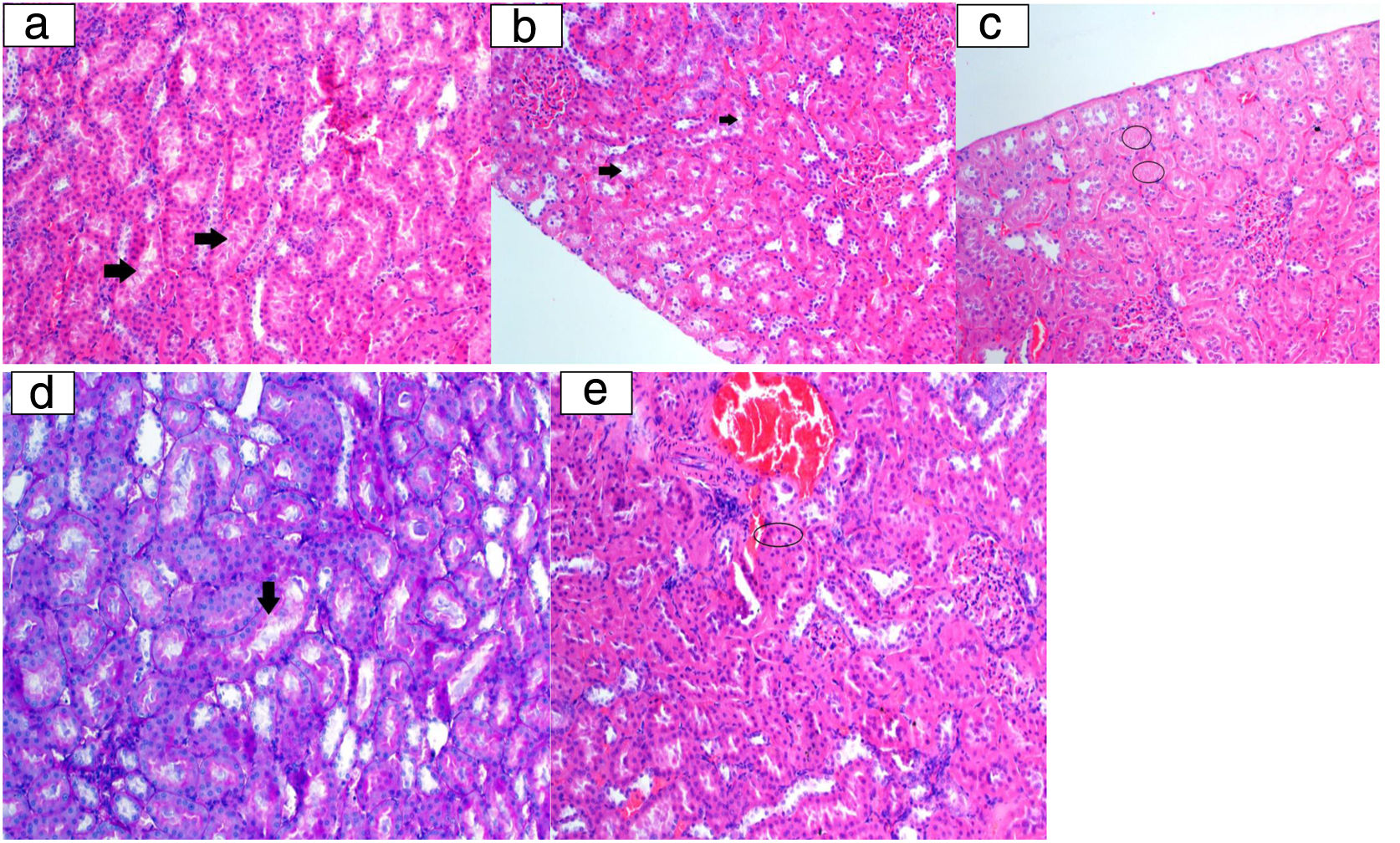
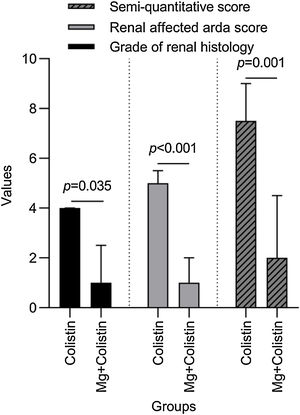
Histopathological findingsGrade of renal histology, renal affected area score, and SQS in rats were significantly lower in the Mg+colistin group compared to the colistin group (p=0.035, p<0.001 and p=0.001, respectively) (Table 2 and Fig. 2). The light microscopic examination showed that the normal structure of the tubules was maintained in the control and Mg groups. Damage to the tubular epithelium was prominent in the colistin group (Fig. 3).
Comparison of histological stages of colistin and Mg-colistin groups.
| Colistin (n=8) | Mg+colistin (n=8) | p-Value | |
|---|---|---|---|
| Grade of renal histology | 4 (2.5–4) | 1 (0.50–2.50) | 0.035 |
| Renal affected area score | 5 (3.50–5.50) | 1 (0.50–2) | <0.001 |
| Semi-quantitative score | 7.50 (7–9) | 2 (1–4.50) | 0.001 |
Values are presented as median (interquartile range).
p-Value shows Mann–Whitney-U test results, a p-value <0.05 was considered significant.
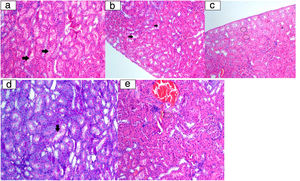
Histopathological examination image. a: Tubular epithelium lumen spill (arrow) (HE 200×); b: vacuolar degeneration in tubulepitel cells (arrow) (HE 200×); c: tubular epithelium necrosis (ring) (HE 200×); d: tubular epithelial cells degeneration at the brushy edge (arrow) (PAS 200×); e: eosinophilic cytoplasm cells (ring) with picnotic nuclei laying tubules (HE 200×).
Colistin has been increasingly used in recent times and is an important nephrotoxic. The present study investigated if the toxic effects of colistin on the kidney decreased when it is combined with the antioxidant mineral Mg. We found that the colistin+Mg group had lower urea and creatinine values and histopathological damage than the Mg group. Additionally, the colistin group had higher plasma MDA and GSH erythrocyte values than the other groups.
Colistin is filtered from glomeruli and secreted from tubules in the kidney.4 Its nephrotoxic effects are observed in the clinic as oliguria, hematuria, acute tubular necrosis.24,25 There are studies showing that oxidative stress plays a role in the formation of nephrotoxicity.9,12,14,19 It causes tubular lysis by causing the entrance of anion, cation, and water through tubular epithelial cell damage and affects in a similar way to bacteriostatic effect. Colistin acts by displacing divalent cations Mg2+ and Ca2+ to disrupt the stability of the bacterial outer membrane. Likewise, it increases the permeability in tubules.26 Many reactive oxygen species that are formed in mitochondria result apoptosis and renal dysfunction. Ozkan et al. determined colistin-related in apoptosis to occur with the activation of caspase I and III.27,28 Many antioxidants have been studied to reduce the nephrotoxicity of colistin.6,10,11,13
Magnesium is a mineral with vital functions such as transport function, enzyme and signaling pathways, energy metabolism, and nucleic acid synthesis.29 It is an antioxidant and anti-inflammatory molecule. It facilitates the intracellular calcium accumulation, which we mentioned in the colistin nephrotoxicity, by inhibiting the l-type calcium channels.30 Magnesium was also found to decrease drug-related cardiotoxicity, apoptosis, and necrosis.31 Moreover, various studies found this molecule to have antioxidant effects. Administration of magnesium sulfate which has an antioxidant effect and dehydration in contrast-associated nephropathy reduced the risk of nephropathy.15 Another study indicated that contrast nephropathy was more common in patients with low magnesium levels.16 There are studies that show the effect of magnesium on nephropathy caused by some chemotherapeutic agents apart from contrast nephropathy. In their experimental study, Saito et al. designed cisplatin nephrotoxicity and showed that giving magnesium to the rats decreased nephrotoxicity by reducing the cisplatin accumulation in the kidney.17 In a study conducted with people who received cisplatin chemotherapy, the supplementation of magnesium to hydration reduced cisplatin nephrotoxicity.18 In another study performed on cyclosporine-treated mice, magnesium supplementation had a renal protective effect by increasing nitric oxide synthase activity in the renal tissue.32
Because there is no study examining the supplementation of Mg with colistin, we referenced other studies for the Mg sulfate doses. In their experiment to reduce the cardiotoxic effects of doxorubicin, Khalilzadeh and colleagues gave rats 120mg/kg for two weeks and magnesium sulfate for three days a week as an IP and found that cardiotoxicity decreased.33 In another study, nephrotoxicity was reduced by administering 40mg/kg Mg sulfate before cisplatin once a week for three weeks in rats given cisplatin.17 However, higher magnesium doses were used in studies that assessed the effects of magnesium in rats with ischemic stroke and in Alzheimer's rat model.34,35
In a review by Heybeli et al. assessing the studies that involved colistin nephrotoxicity in rats, it was observed that colistin was given at 300,000–480,000IU/kg/day or 15–18mg/kg/day or 36–84mg/kg cumulative doses because five of the eleven studies gave 300,000IU/kg/day. In their article which was conducted in 2019 and included views of many institutions on the use of polymyxin, Tsuji et al. suggested 9,000,000IU CMS maintenance dose to be systemically administered every 12–24h after the 9,000,000IU CMS loading dose and this was significantly lower than the dose (300,000IU/kg/day) we administered in the present study.36 The drug was provided for seven days in the majority of the studies.37 In their study in which they administered 300,000IU/day CMS for six days, Ozkan and Ozyılmaz determined that colistin caused a significant level of toxicity in the tissue and biochemical levels of the rats.28,38 Based on this study, we administered colistin at a dose of 300,000IU/kg/day, and for seven days.
We found that urea value decreased significantly in the Mg and Mg+colistin group, creatinine value increased in the colistin and Mg groups and there was no significant increase in the control and Mg+colistin groups. Although the decrease in the urea value of the Mg and Mg+colistin group supported the renal protective effect of magnesium, it was also interesting to find that creatinine levels increased in the Mg group. Cheungpasitporn et al. showed that both hypomagnesemia and hypermagnesemia were associated with an increased risk of acute kidney disease. They considered that overstimulated vasodilatation and vasoconstriction may be effective.39 Hypermagnesemia may occur in the group that was administered only magnesium and may cause creatinine value to be higher than the baseline. The absence of histopathological changes in this group receiving magnesium alone may support the idea that a prerenal event may trigger an increase in creatinine. Severe hypomagnesemia was detected in the colistin-treated groups in two studies in patients who were given colistin in the neonatal intensive care unit.40,41 Hypomagnesemia in the colistin group may lead to increased oxidative stress leading to renal toxicity. Reduction of renal toxicity by adding magnesium to colistin may be a result of regulating hypomagnesemia with colistin.
MDA causes serious damage to the cell membrane structure.42 GSH is a tripeptide that reacts with hydrogen peroxide and organic acids and shows an antioxidant effec.43 In the study by Edrees et al., concurrent administration of curcumin with colistin led to a decrease in the MDA level and an increase in GSH level in renal tissue. Lee, Özkan, and Ghlissi found that some antioxidant molecules used in their studies decreased the MDA levels compared to the colistin group.7,14,44 Ozyilmaz et al. found no change in the MDA level in plasma in the group given N-acetylcysteine together with colistin.38 We found that plasma MDA levels were significantly increased in the colistin group compared to the beginning of the experiment and compared to the other groups and decreased in the Mg and Mg+colistin groups. While the colistin group had significantly lower erythrocyte GSH levels than the other groups, Mg+colistin group was higher than the colistin group, however; it was not statistically significant. Although renal GSH levels were the lowest in the colistin group renal MDA levels were the highest in the colistin group, but it was not statistically significant.
We found tubular injury, renal involvement area, and SQS were found to be high in the colistin-treated group, while the histopathological changes were significantly decreased in the magnesium-treated and colistin-treated rats. This was in line with previous studies.9,10,13
There were some limitations to our study. We did not study the effects of different doses of magnesium and colistin. The fact that we did not study serum magnesium levels at the beginning and end of the study was another limitation of this study. However, this study was the first to examine colistin nephrotoxicity with magnesium which is an easily accessed, inexpensive molecule with many functions in the body. We believe that comprehensive and more detailed studies should be conducted to investigate the relationship between magnesium and colistin nephrotoxicity.
Ethics standardsThe study was approved by the Ethics Advisory Committee Selçuk University Experimental Medicine Application and Research Center (IRB approval number: 2019-46). All procedures performed in animal studies were in accordance with the ethical standards of the institution or practice in which the studies were conducted.
Conflict of interestThe authors have declared that no conflict of interest exists.