Adequate serum phosphorus levels in patients with chronic kidney disease is essential for their clinical management. However, the control of hyperphosphatemia is difficult because is normally associated with increases in serum PTH. In the present study, the effects of hyperphosphatemia, in the presence of elevated and normal PTH, on cardiac inflammation, hypertrophy and fibrosis in an experimental renal failure model were analyzed.
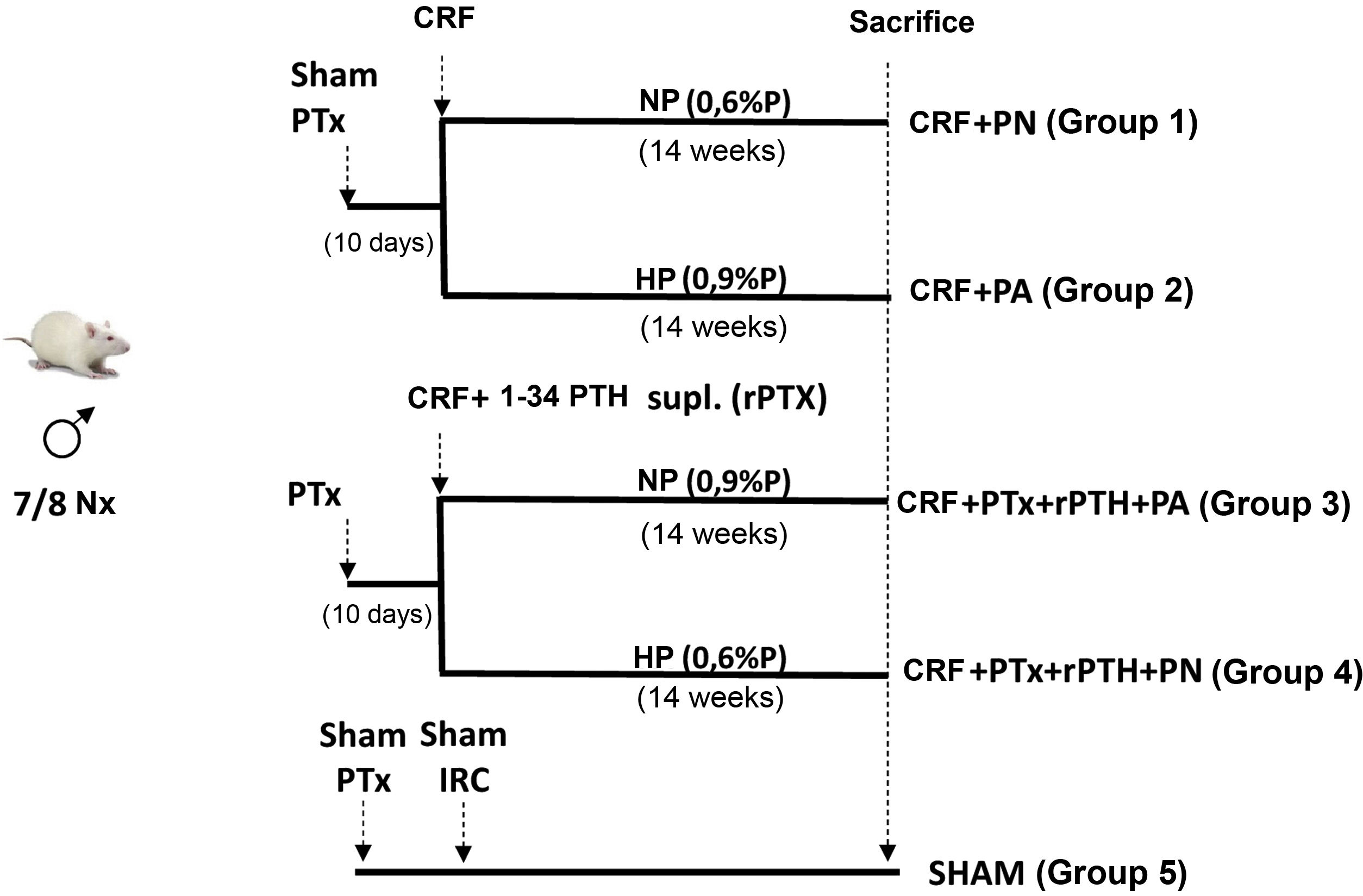
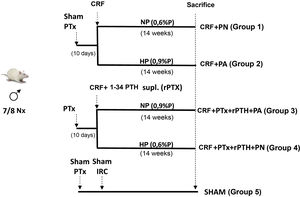
Materials and methods4 groups of rats were formed. Two groups underwent total parathyroidectomy (PTx). Rats with Ca <7.5 mg/dL and PTH < 50 pg/mL underwent 7/8 nephrectomy (CRF) and a subcutaneous pellet was placed that releases PTH 1-34 (5 µg/kg/day). One group received a diet with normal P (NP) (CRF + PTx + rPTH + NP group) and another with a high P diet (0.9% - HP) (CRF + PTx + rPTH + HP group). Other 2 groups that only had CRF received NP (CRF + NP) and HP (CRF + HP) diet. A SHAM group for nephrectomy and parathyroidectomy was also added. After 14 weeks the rats were sacrificed.
ResultsThe groups with a diet high in phosphorus (CRF + H A and CRF + PTx + rPTH + HP) had a significant reduction in creatinine clearance and also in body weight with an increase in serum phosphorus regardless of parathyroidectomy, but not serum levels of calcium, FGF23 and calcitriol that were 2–3 times higher in the group with secondary hyperparathyroidism (CRF + HP).
The diameter of the cardiomyocytes was greater in the CRF + HP group, while parathyroidectomy (CRF + PTx + rPTH + HP) significantly reduced them, despite the high and similar serum phosphorus values. TNF-α, Adam17 and cardiac fibrosis at the histological and molecular level showed a similar pattern with increases in the group with severe secondary hyperparathyroidism (CRF + HP).
ConclusionsHyperphosphatemia confirmed its importance in the genesis of secondary hyperparathyroidism, but also of kidney damage that was independent of PTH levels. However, inflammation, fibrosis, and cardiomyocyte growth were more closely related to PTH levels, since in the presence of similar severe hyperphosphatemia, parathyroidectomy reduced the values of inflammatory parameters, cardiac hypertrophy, and fibrosis.
Mantener niveles adecuados de fósforo sérico en el paciente con enfermedad renal crónica es fundamental para su correcto manejo clínico. Sin embargo resulta difícil su control de forma aislada porque normalmente se asocian con aumentos séricos de PTH. En el presente estudio se analizaron los efectos de la hiperfosfatemia aislada, en presencia de PTH elevada y normal, sobre la inflamación, hipertrofia y fibrosis cardiaca en un modelo de insuficiencia renal experimental.
Materiales y métodosSe formaron 4 grupos de ratas. A dos grupos se les realizó paratiroidectomía total (PTx). A las ratas con Ca < 7,5 mg/dL y PTH < 50 pg/mL se les realizó nefrectomía 7/8 (IRC) y se les colocó un pellet subcutáneo que libera PTH 1-34 (5 µg/kg/día). Un grupo recibió dieta con P normal (PN) (grupo IRC + PTx + rPTH + PN) y otro dieta con P alto (0,9%-PA) (grupo IRC + PTx + rPTH + PA). Otros 2 grupos que solo tenían IRC recibieron dieta PN (IRC + PN) y PA (IRC + PA). Se añadió también un grupo SHAM para nefrectomía y paratiroidectomía. Tras 14 semanas las ratas fueron sacrificadas.
ResultadosLos grupos con dieta alta en fósforo (IRC + PA e IRC + PTx + rPTH + PA) tuvieron una reducción significativa del aclaramiento de creatinina y también del peso corporal con un aumento del fósforo sérico independientemente de la paratiroidectomía, pero no así los niveles séricos de calcio, FGF23 y de calcitriol que fueron 2–3 veces superiores en el grupo con hiperparatiroidismo secundario (IRC + PA). El diámetro de los cardiomiocitos fue superior en el grupo IRC + PA, mientras la paratiroidectomía (IRC + PTx + rPTH + PA) los redujo significativamente, a pesar de los elevados y similares valores de fósforo sérico. El TNF-α, Adam17 y la fibrosis cardiaca a nivel histológico y molecular mostraron un patrón similar con aumentos en el grupo con hiperparatiroidismo secundario severo (IRC + PA).
ConclusionesLa hiperfosfatemia confirmó su importancia en la génesis del hiperparatiroidismo secundario, pero también del daño renal que fue independiente de los niveles de PTH. Sin embargo, la inflamación, fibrosis y crecimiento de cardiomiocitos guardaron una mayor relación con los niveles de PTH ya que en presencia de hiperfosfatemia severa similar, la paratiroidectomía redujo los valores de los parámetros inflamatorios, de hipertrofia y fibrosis cardiaca.
The control of serum phosphorus levels has always been recognized as a key element in the clinical management of patients with chronic kidney disease (CKD).1 In advanced CKD, renal phosphorus excretion is insufficient to eliminate the amount of phosphorus that is absorbed daily from the diet; consequently, hyperphosphatemia develops.2 Alterations in phosphorus metabolism are associated with important adverse consequences such as progression of secondary hyperparathyroidism, more rapid progression of chronic kidney disease, and increases in mortality.2–4
Since hyperphosphatemia and hyperparathyroidism usually coincide, it is difficult to assess the isolated effect of these two factors. Hyperphosphatemia has been associated with pericarditis, hypertension and left ventricular hypertrophy5 and in rats with chronic renal failure it has been associated with cardiomyocyte hypertrophy and apoptosis, increased myocardial fibrosis6,7 and inflammation.8
Similarly, elevated PTH levels have shown a direct hypertrophic effect in cardiomyocytes isolated from adult rats.9 There are several studies on the association between secondary hyperparathyroidism and left ventricular hypertrophy in patients with advanced chronic kidney disease.10,11 Secondary hyperparathyroidism has also been associated with the development of cardiac fibrosis,12 however there are limited data regarding the role that elevated PTH levels could have in the development of the inflammatory process that would precede fibrosis.
In addition, parathyroidectomy has been associated with a regression of ventricular hypertrophy and improvement in cardiac contractility in hemodialysis patients.13 In rats with chronic renal failure, parathyroidectomy prevented cardiac hypertrophy, fibrosis, and cell apoptosis.14
In the present study evaluates, the effects of isolated hyperphosphatemia in the presence of elevated or normal PTH on cardiac inflammation, hypertrophy and fibrosis.
Material and methodsExperimental modelThirty four-month-old male Wistar rats (weight 399 ± 46 g) from the University of Oviedo Animal Facility were used for the study. The protocol is described in Fig. 1. Parathyroidectomy (PTx) was performed in a number of rats and those animals with a serum concentration of total calcium (Ca) < 7.5 mg/dL and PTH (PTH1−84) <50 pg/mL were considered optimal to continue in the study. Ten days after parathyroidectomy, chronic renal failure (CRF) was induced by partial nephrectomy, consisting of total removal of the right kidney and section of the lower and upper poles and part of the renal parenchyma, leaving approximately 1/8 of a renal mass.15 Simultaneously with the nephrectomy, physiological pellets were subcutaneously implanted in PTx rats for continuous infusion of 5 μg/kg/day PTH 1-34 (rPTH) (Innovative Research of America®, FL, USA). This dose was chosen based on other previous studies to achieve a physiological replacement of PTH after parathyroidectomy.12,16 In a group of rats with nephrectomy and without parathyroidectomy, subcutaneous pellets with vehicle were also implanted (Sigma-Aldrich®, MO, USA). A SHAM group, sham nephrectomy and parathyroidectomy, was added as a comparison group, sham rats were fed a normal phosphorus diet and received vehicle through subcutaneous pellets (Sigma-Aldrich®).
All CRF rats (with or without PTx) were divided according to the diet administered: normal phosphorus diet (NP) (0.6% P and 0.6% Ca) (Panlab, Spain) and high phosphorus diet (HP) (0.9% P and 0.6% Ca) (Panlab) and were housed in cages receiving food and water ad libitum. After 14 weeks, prior to sacrifice, rats were placed in metabolic cages during 24 h for urine collection.
Sacrifice was performed by exsanguination using isoflurane anesthesia and serum samples were taken for analysis. The heart was removed and washed twice with saline solution to remove some clots that could remain after exsanguination, then it was dried thoroughly with a filter paper, weighed and processed for histological studies and RNA extraction.
Biochemical markersTotal calcium, phosphorus, total protein, urea, and creatinine were determined in serum and urine using an automated multichannel analyzer (Hitachi 717®, Boehringer Mannheim, Germany). Intact serum PTH (1−84) and intact FGF23 were measured by an ELISA sandwich (Immunotopics®, USA and Kainos Laboratories®, Japan) and PTH (1−34) was measured by a RIA immunoassay (Phoenix Pharmaceutical® Inc, USA).
Morphological and histological changes and quantification of collagen 1Masson's trichrome stain was used in cardiac tissue with which the collagen I fibers of the connective tissue are highlighted in blue, also staining the rest of the tissue structures. The total content of collagen I in the myocardium was determined by measuring the area of stained collagen obtained by a semi-automatic image obtained with a Leica DMRXA2 microscope (Leica), coupled to a camera Leica DFC7000T (Leica). The measurements were blind and the results were expressed as percentages of blue stained area in relation to the total area of the myocardium. The captured images were analyzed using image analysis (Leica Q500IW®, Leica Microsystems) and specific software (Leica QWIN® standard version 2.3, Leica Microsystems).
Thickness of the wall and ventricular septum; size of cardiomyocytesHematoxylin-eosin staining was used to quantify the thickness of the wall and septum, and the diameter of cardiomyocytes of the left ventricle using the same optical system described previously in deparaffinized sections and stained with hematoxylin-eosin. The captured images were analyzed using the same imaging system mentioned above.
The mean diameter of the cardiomyocyte was determined by randomly measuring the width of the cardiomyocyte at the center of the nucleus in 20 cardiomyocytes, as has been described by other authors.17,18 To make these measurements we selected the same area of the left ventricle using the lowest magnification of the microscope, selecting those cardiomyocytes hat were in the same plane, with its nucleus centered. To quantify the thickness of the wall and septum, a software previously design was used that groups and analyzes a set of at least 50 measurements from the left ventricular pillars to the outer edge, in the case of the wall, and the edge between the left ventricle. and right in case of the septum.
Molecular markers of inflammation and fibrosisThe gene expression of tumor necrysis factor alpha (TNF-α) and domain 17 of the metallopeptidase Adam (Adam17) were analyzed as molecular markers of inflammation. As molecular markers of cardiac fibrosis we analyzed the gene expression of collagen type 1, the tumor necrosis factor beta 1 (TGF-β1), fibronectin and connective tissue growth factor (CTGF). To perform these studies, RNA was extracted and gene expression was analyzed by quantitative real-time PCR (qPCR). A fragment of the heart (left ventricle) of the rats was homogenized in an ultraturrax (OmniHT) in TRI reagent (Sigma-Aldrich, USA), following the manufacturer's instructions. The total RNA concentration and purity were quantified by UV–vis spectrophotometry (NanoDrop Technologies®) measuring its absorbance at 260 and 280 nm. The copy DNA (cDNA) was obtained with a high capacity cDNA reverse transcription kit (Applied Biosystems®, USA) following the manufacturer's instructions. Gene expression was measured by qPCR using a Stratagene Mx 3005P® kit (Agilent Technologies). Real-time PCR amplification was done using TaqMan probes, performed with gene-specific primers (Applied Biosystems® gene expression assays). GAPDH was used as housekeeping gene. The relative quantitative evaluation of the target genes was carried out by comparing the threshold cycles using the ⊗⊗Ct19 method. The results were represented as relative units (RU) with respect to group 1 (CRF + NP) that was adjusted to the unit. The protocol was approved by the Research Ethics Committee of the University of Oviedo.
Statistical analysisThe non-parametric Kruskal Wallis test with Bonferroni post-hoc analysis was used to compare independent samples. The results were expressed as median and interquartile range. Differences were considered significant if P < .05. The R 3.5.0 was the statistical program used in this study.
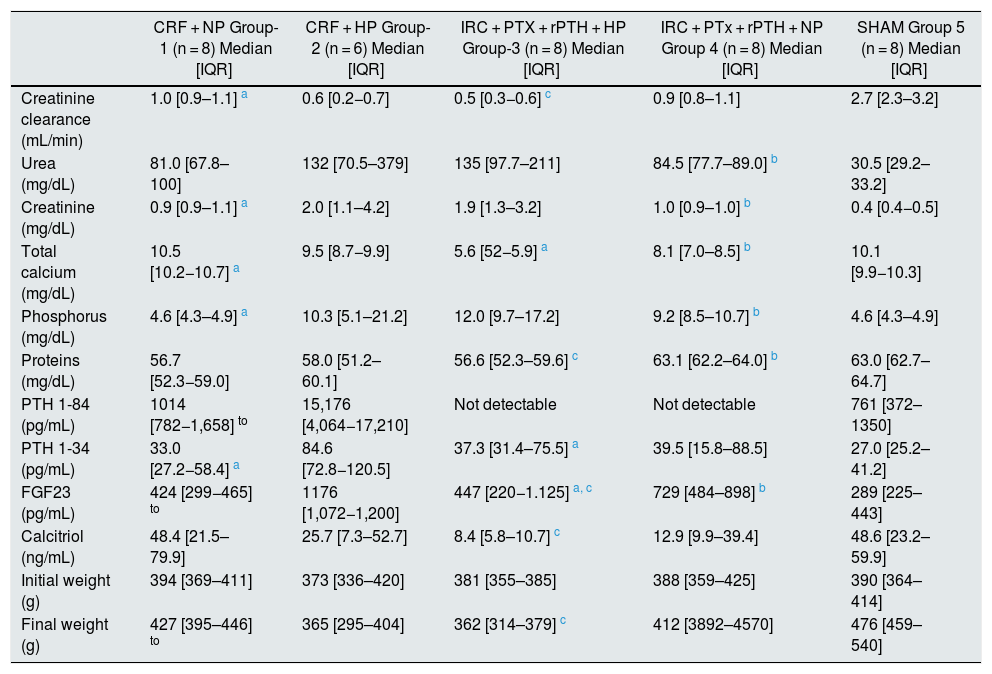
ResultsBiochemical, morphological and histological markersParameters of Biochemistry are shown in Table 1. Groups 2 and 3 on a high phosphorus diet (CRF + HP and CRF + PTx + rPTH + HP) had a significant reduction in creatinine clearance regardless whether they had parathyroidectomy (groups 3 and 4). In both groups, the serum phosphorus levels were not significantly different, however total serum calcium did. Despite no significant differences in hyperphosphatemia, serum levels of FGF23 were three times higher in the group with secondary hyperparathyroidism (CRF + HP, group 2). The parathyroidectomized rats (groups 3 and 4) showed serum levels of PTH 1-34 similar to those of group 1 and these were slightly lower than the group 2 with severe secondary hyperparathyroidism, although the difference was not significant. There were no differences in the initial weight between any of the four groups, however, there were differences in the final weight. In rats fed high phosphorus (groups 2 and 3), in addition to hyperphosphatemia, the weight was reduced as compared to the control group (group 1) and to the group with a normal phosphorus diet (group 4), these variations were independent of parathyroidectomy.20
Biochemical parameters in 7/8 nephrectomy rats fed a diet with normal phosphorus content (0.6% NP) or high phosphorous content (0.9% HP) with and without parathyroidectomy.
| CRF + NP Group-1 (n = 8) Median [IQR] | CRF + HP Group-2 (n = 6) Median [IQR] | IRC + PTX + rPTH + HP Group-3 (n = 8) Median [IQR] | IRC + PTx + rPTH + NP Group 4 (n = 8) Median [IQR] | SHAM Group 5 (n = 8) Median [IQR] | |
|---|---|---|---|---|---|
| Creatinine clearance (mL/min) | 1.0 [0.9–1.1] a | 0.6 [0.2−0.7] | 0.5 [0.3−0.6] c | 0.9 [0.8–1.1] | 2.7 [2.3–3.2] |
| Urea (mg/dL) | 81.0 [67.8–100] | 132 [70.5–379] | 135 [97.7–211] | 84.5 [77.7–89.0] b | 30.5 [29.2–33.2] |
| Creatinine (mg/dL) | 0.9 [0.9–1.1] a | 2.0 [1.1–4.2] | 1.9 [1.3–3.2] | 1.0 [0.9–1.0] b | 0.4 [0.4−0.5] |
| Total calcium (mg/dL) | 10.5 [10.2−10.7] a | 9.5 [8.7−9.9] | 5.6 [52−5.9] a | 8.1 [7.0–8.5] b | 10.1 [9.9−10.3] |
| Phosphorus (mg/dL) | 4.6 [4.3–4.9] a | 10.3 [5.1–21.2] | 12.0 [9.7–17.2] | 9.2 [8.5–10.7] b | 4.6 [4.3–4.9] |
| Proteins (mg/dL) | 56.7 [52.3−59.0] | 58.0 [51.2–60.1] | 56.6 [52.3–59.6] c | 63.1 [62.2–64.0] b | 63.0 [62.7–64.7] |
| PTH 1-84 (pg/mL) | 1014 [782−1,658] to | 15,176 [4,064−17,210] | Not detectable | Not detectable | 761 [372–1350] |
| PTH 1-34 (pg/mL) | 33.0 [27.2−58.4] a | 84.6 [72.8−120.5] | 37.3 [31.4–75.5] a | 39.5 [15.8–88.5] | 27.0 [25.2–41.2] |
| FGF23 (pg/mL) | 424 [299−465] to | 1176 [1,072−1,200] | 447 [220−1.125] a, c | 729 [484–898] b | 289 [225–443] |
| Calcitriol (ng/mL) | 48.4 [21.5–79.9] | 25.7 [7.3–52.7] | 8.4 [5.8–10.7] c | 12.9 [9.9–39.4] | 48.6 [23.2–59.9] |
| Initial weight (g) | 394 [369–411] | 373 [336–420] | 381 [355–385] | 388 [359–425] | 390 [364–414] |
| Final weight (g) | 427 [395–446] to | 365 [295–404] | 362 [314–379] c | 412 [3892–4570] | 476 [459–540] |
CRF + NP (group 1), 7/8 nephrectomy on a normal phosphorous diet; CRF + HP (group 2), 7/8 nephrectomy on a high phosphorus diet; CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and on a high phosphorus diet; CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and normal phosphorus diet; SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
The data are represented as the median and the interquartile range [IQR].
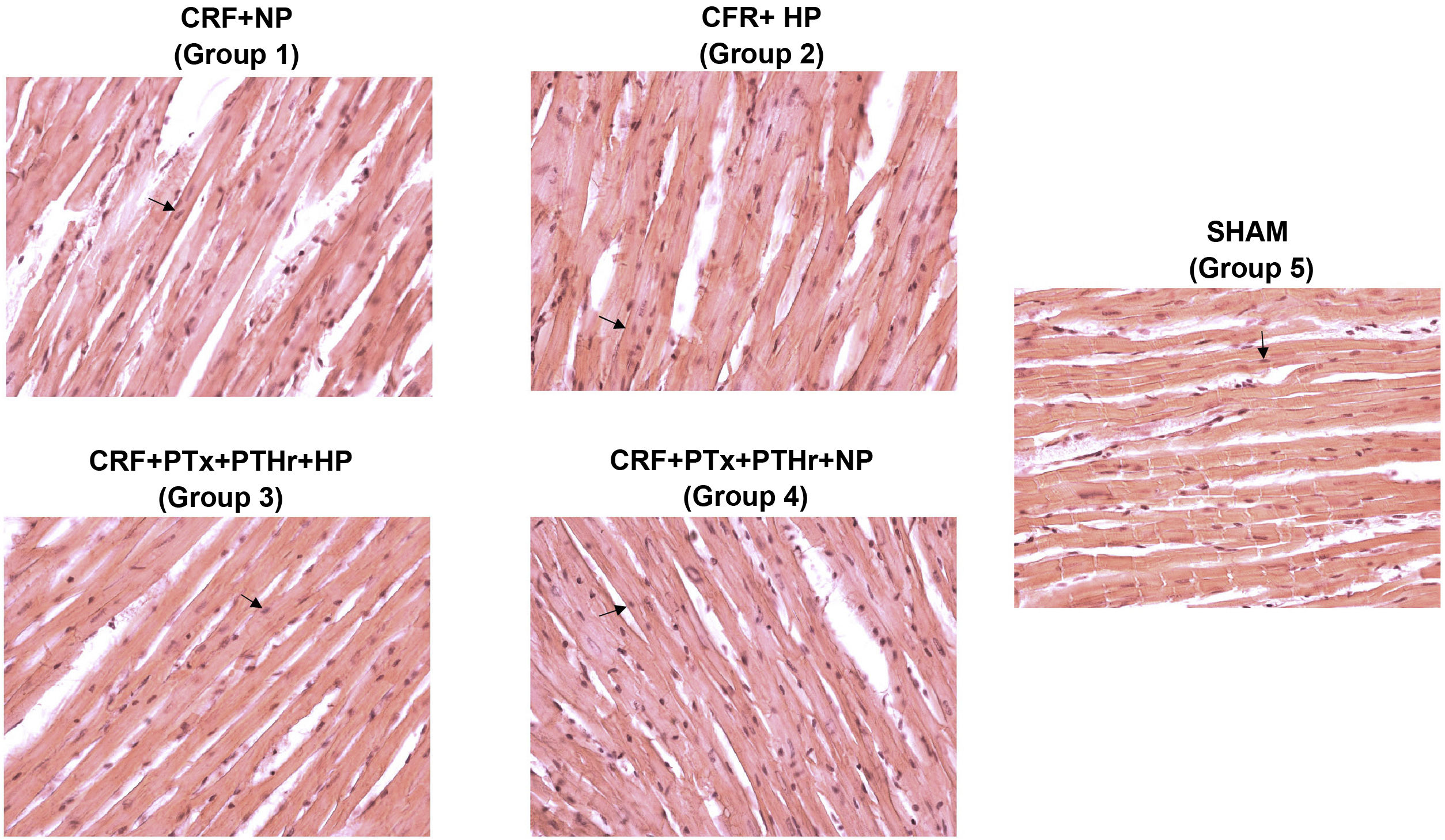
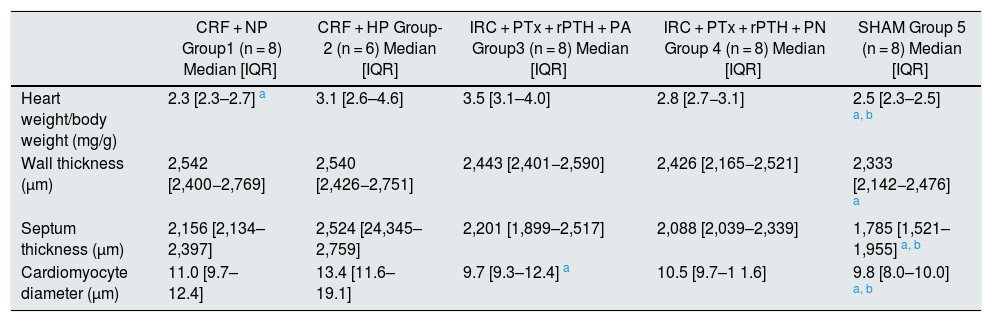
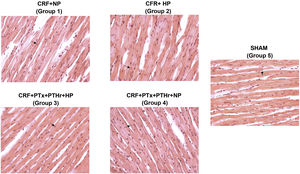
In rats without parathyroidectomy the heart weight relative to body weight was significantly greater in rats fed a high phosphorus diet (CRF + HP) (group 2) than their control group on a normal phosphorus diet (CRF + PN) (group 1). The thickness of the wall and septum did not show significant differences between the groups, but the diameter of the cardiomyocytes was increased in group 2 (CRF + HP) as compared with group 1. The highest values of diameter of the cardiomyocytes were observed in group 2. Parathyroidectomy (CRF + PTx + rPTH + HP) significantly reduced (Table 2, and Fig. 2). There were no differences in any marker of cardiac hypertrophy between the CRF + NP and CRF + PTx + rPTH + NP groups (groups 1 and 4).
Parameters of cardiac hypertrophy in rats with 7/8 nephrectomy fed a normal phosphorus diet (0.6% NP) or a high phosphorous diet (0.9% HP) with and without parathyroidectomy.
| CRF + NP Group1 (n = 8) Median [IQR] | CRF + HP Group-2 (n = 6) Median [IQR] | IRC + PTx + rPTH + PA Group3 (n = 8) Median [IQR] | IRC + PTx + rPTH + PN Group 4 (n = 8) Median [IQR] | SHAM Group 5 (n = 8) Median [IQR] | ||
|---|---|---|---|---|---|---|
| Heart weight/body weight (mg/g) | 2.3 [2.3–2.7] a | 3.1 [2.6–4.6] | 3.5 [3.1–4.0] | 2.8 [2.7−3.1] | 2.5 [2.3–2.5] a, b | |
| Wall thickness (μm) | 2,542 [2,400−2,769] | 2,540 [2,426−2,751] | 2,443 [2,401−2,590] | 2,426 [2,165−2,521] | 2,333 [2,142−2,476] a | |
| Septum thickness (μm) | 2,156 [2,134–2,397] | 2,524 [24,345–2,759] | 2,201 [1,899–2,517] | 2,088 [2,039–2,339] | 1,785 [1,521–1,955] a, b | |
| Cardiomyocyte diameter (μm) | 11.0 [9.7–12.4] | 13.4 [11.6–19.1] | 9.7 [9.3–12.4] a | 10.5 [9.7–1 1.6] | 9.8 [8.0–10.0] a, b | |
CRF + NP (group 1), 7/8 nephrectomy on a normal phosphorous diet; CRF + HP (group 2), 7/8 nephrectomy on a high phosphorus diet; CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and on a high phosphorus diet; CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and normal phosphorus diet; SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
Data are represented as the median and interquartile range [IQR].
Representative images of hematoxylin-eosin staining of cardiomyocytes in each group (40× magnification).
CRF + NP (group 1), 7/8 nephrectomy on a normal phosphorous diet; CRF + HP (group 2), 7/8 nephrectomy on a high phosphorus diet; CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy, PTH replacement on a high phosphorus diet; CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy and on a normal phosphorous diet; SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
The analysis of the correlations between biochemical parameters such as PTH, FGF23 and calcitriol with the markers of cardiac hypertrophy show that the thickness of the septum correlated with the serum levels of intact PTH (r = 0.649, P < .001) and FGF23 (r = 0.516), P = .008). Cardiomyocyte diameter also correlated with intact PTH levels (r = 0.707, P < .001).
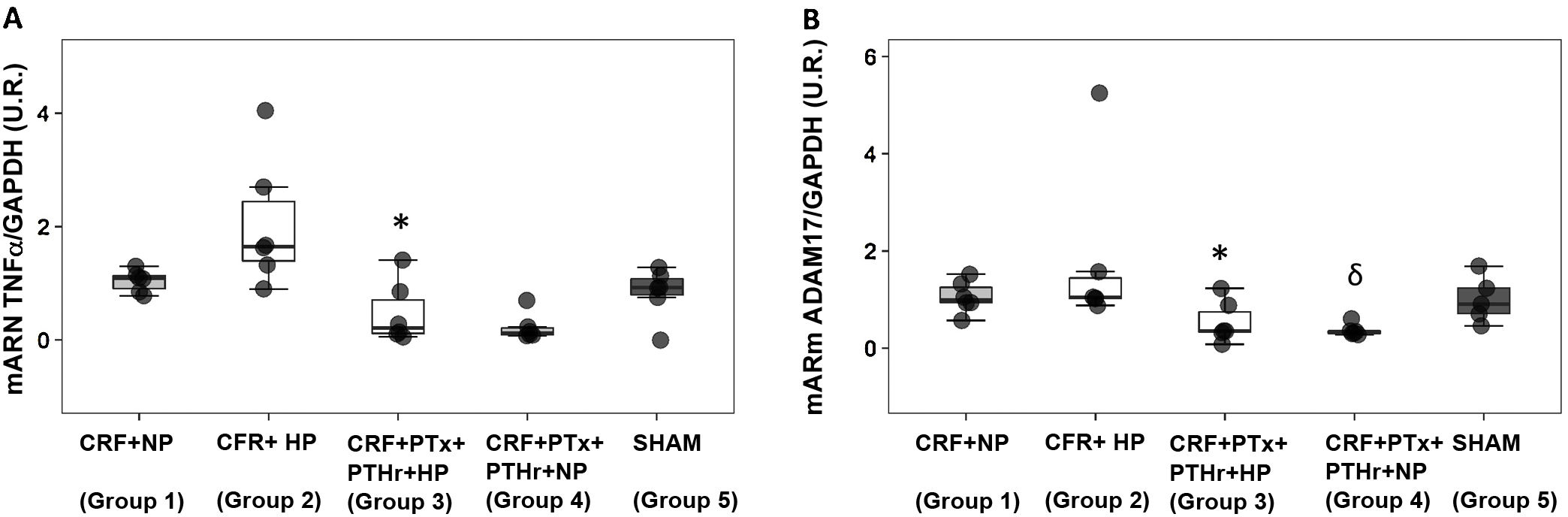
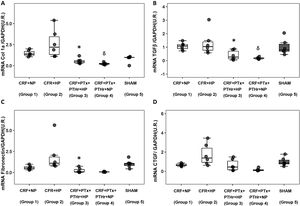
Molecular markers of inflammation and fibrosisGene expression of TNF-α followed a pattern very similar to that observed in the size of cardiomyocytes. Group 2 (CRF + HP) showed the higher values as compared to group 3(CRF + PTx + rPTH + PA). The expression of Adam17 showed a similar pattern as TNF-α. Parathyroidectomy significantly reduced TNF-α and Adam17 despite the high serum phosphorus levels observed in group 3 (Fig. 3, Table 1).
Molecular markers of inflammation. Box plot graph. A) mRNA TNF-α (UR), B) mRNA Adam17 (UR) in heart of 7/8 nephrectomy rats fed a diet containing normal phosphorus (0.6% NP) or high phosphorous ( 0.9% HP) with and without parathyroidectomy.
CRF + NP (Group 1): 7/8 nephrectomy on a normal phosphorous diet.
CFR + HP (Group 2) 7/8 nephrectomy on a high phosphorus diet.
CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy, PTH replacement on a high phosphorus diet.
CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy on a normal phosphorous diet.
SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
The data are represented as median and the interquartile range.
*P < .05 vs. CRF + HP
δP < .05 vs. CRF + NP.
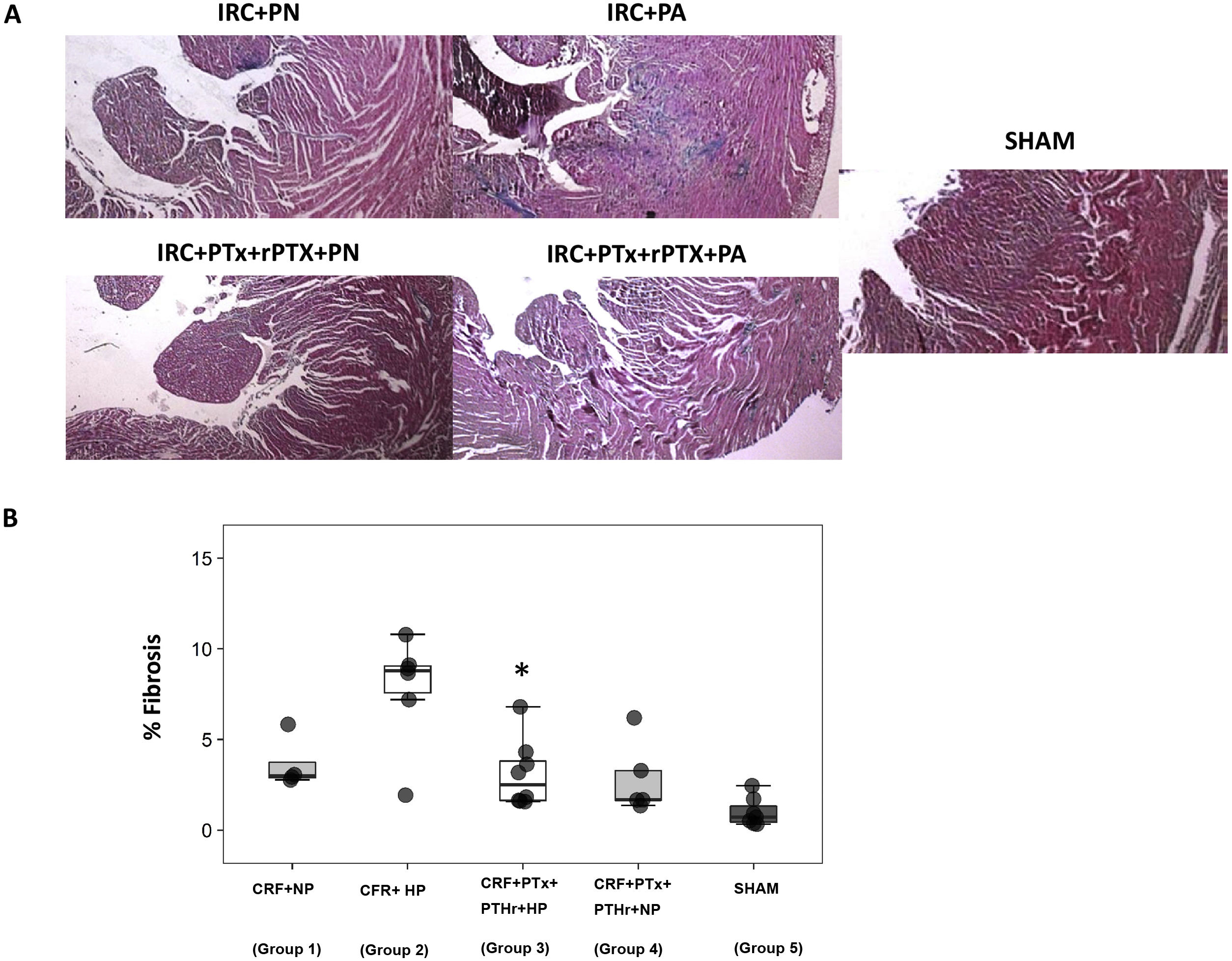
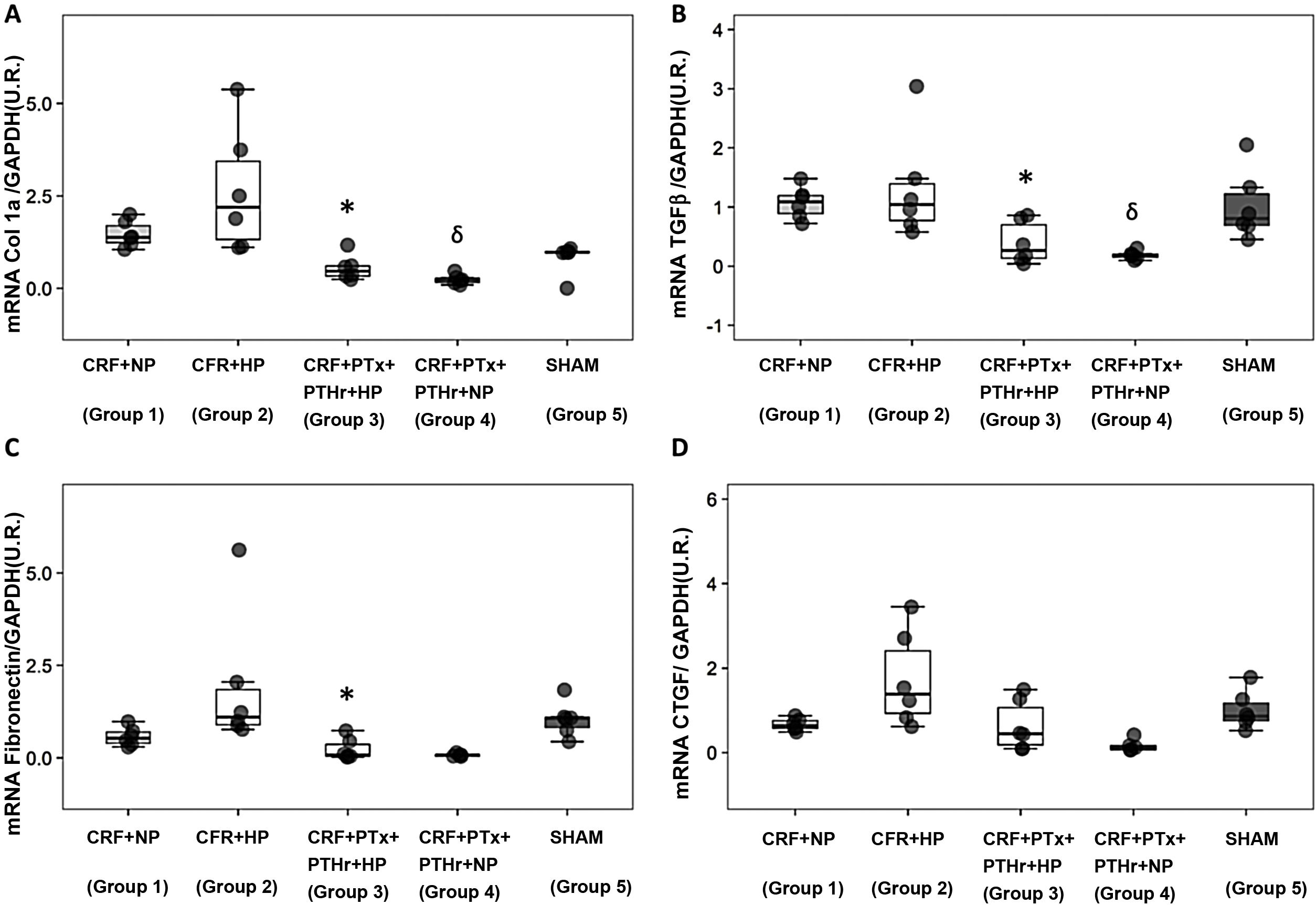
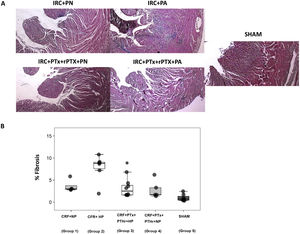
The above pattern was repeated in the analysis of cardiac fibrosis. Group2 (CRF + PA) showed more cardiac fibrosis than group 3 (CRF + PTx + rPTH + PA) (Fig. 4 A and B). Molecular markers of fibrosis (type 1 collagen, fibronectin, TGF-β1 and CTGF) presented a pattern that paralleled morphological and histological changes (Fig. 5). Group 2 (CRF + BP) showed the highest values of molecular markers of inflammation and fibrosis. The removal of parathyroid glands (group 3) reduced these markers despite the presence of hyperphosphatemia. Similarly, in rats from groups 1 and 4 that were all fed a normal phosphorus diet, the molecular markers of inflammation and fibrosis were reduced in group 4 that underwent PTX as compared with group 1 with intact parathyroid glands.
Interstitial cardiac fibrosis. A) Representative images of Masson's trichrome stain in each group (10× magnification). B) Box plot of interstitial cardiac fibrosis measured by Masson's trichrome stain in the heart of rats with 7/8 nephrectomy fed a diet with normal phosphorus content (0.6% NP) or with a high phosphorus content (0.9% HP) with and without parathyroidectomy.
CRF + NP (Group 1): 7/8 nephrectomy on a normal phosphorous diet.
CFR + HP (Group 2) 7/8 nephrectomy on a high phosphorus diet.
CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy, PTH replacement on a high phosphorus diet.
CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy on a normal phosphorous diet.
SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
The data are represented as median and the interquartile range.
*P < .05 vs. CRF + HP.
Molecular markers of cardiac fibrosis. Box plot graph A) Collagen 1 mRNA (UR), B) TGF-β1 mRNA (UR), C) Fibronectin mRNA (UR), D) CTGF mRNA (UR) in the heart of 7/8 nephrectomy rats fed a diet with normal phosphorus content (0.6% PN) or high phosphorous content (0.9% PA) with and without parathyroidectomy.
CRF + NP (group 1), 7/8 nephrectomy on a normal phosphorous diet; CRF + HP (group 2), 7/8 nephrectomy on a high phosphorus diet; CRF + PTx + rPTH + HP (group 3), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and on a high phosphorus diet; CRF + PTx + rPTH + NP (group 4), 7/8 nephrectomy with parathyroidectomy + PTH 1-34 replacement and normal phosphorus diet; SHAM (group 5), sham intervention for nephrectomy and parathyroidectomy.
The data are represented as the median and the interquartile range.
*P < .05 vs. CRF + HP.
δP < .05 vs. CRF + NP.
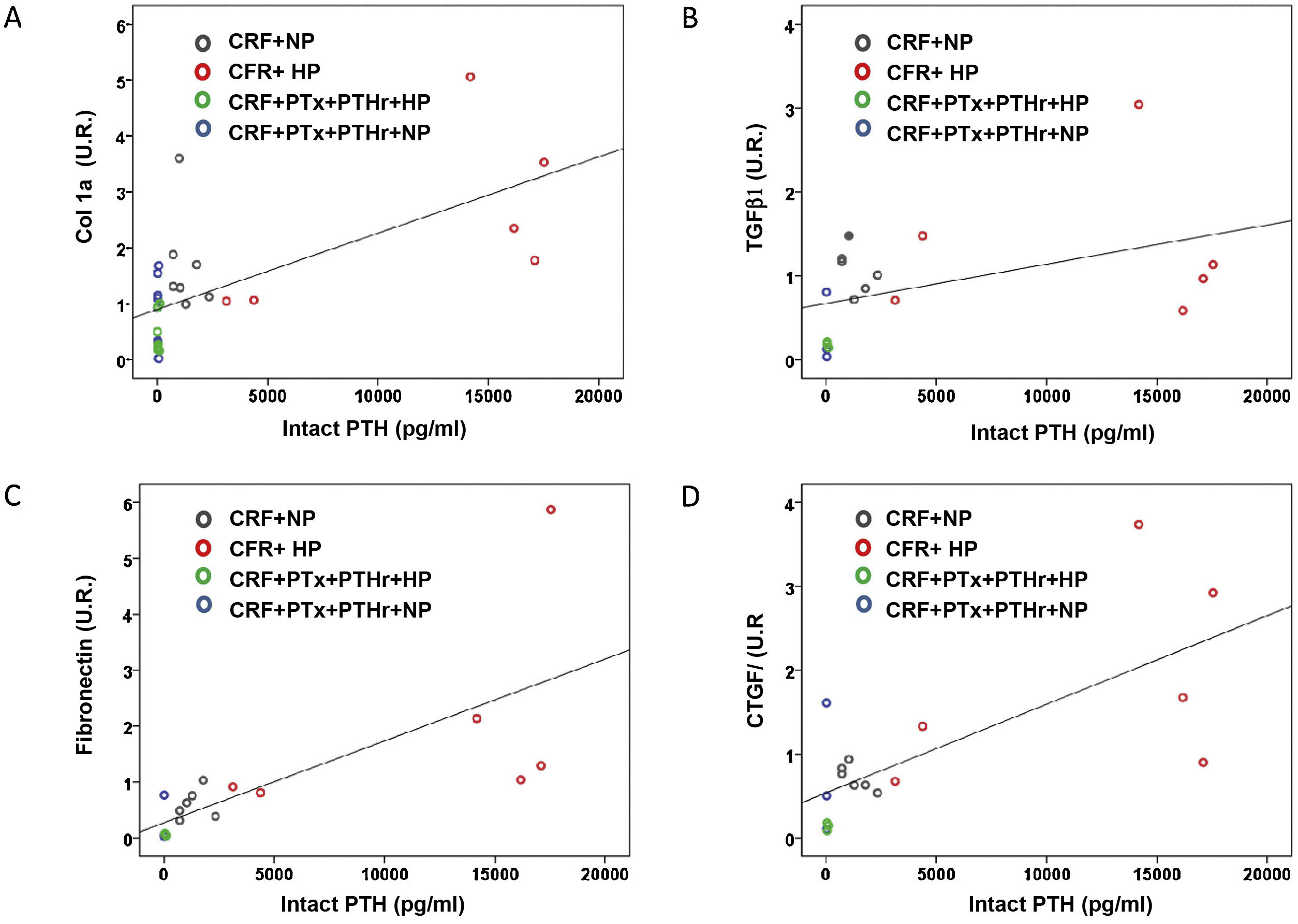
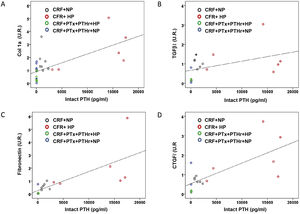
Correlation analysis between biochemical parameters such as PTH, FGF23 and calcitriol with the markers of cardiac fibrosis show that only intact PTH serum levels were positively and significantly associated with cardiac fibrosis (r = 0.590, P = .006). The concentration of PTH was also significantly correlated with the rest of the molecular markers of cardiac fibrosis (Fig. 6): type 1 collagen (r = 0.660, P < .001); TGF-β1 (r = 0.436, P = .048); fibronectin (r = 0.757, P = .001) and CTGF (r = 0.718, P = .001).
Correlations between molecular markers of cardiac fibrosis and serum levels of intact PTH. Simple scatter plot of the correlations of intact PTH serum levels with A) collagen 1 mRNA (UR), B) TGF-βI mRNA (UR), fibronectin mRNA (UR), D) CTGF mRNA (UR) in hearts of rats from all treatment groups.
The TRCP6 calcium channel receptor mRNA levels showed an increase in the CRF + HP group compared to the CRF + PTx + rPTH + HP group (1.75 [1.19–2.09] as compared to 0.82 [0, 12−1.08], P < .05). The groups of rats fed a normal phosphorus diet did not show significant differences in the expression of TRCP6, although the lower levels were observed in parathyroidectomized rats (CRF + NP: 1.06 [0.81–1.88]; CRF + PTx + rPTH + NP: 0, 58 [0.06–1.10]).
DiscussionIn the present study it was observed that the high phosphorus diet contributed to the decrease in creatinine clearance independently of PTH.20,21 Recently published data20 showed that with a similar renal function and comparable serum phosphorus levels, the presence of severe secondary hyperparathyroidism (CRF + HP), but not moderate secondary hyperparathyroidism (CRF + NP), was the factor that more seemed to influence cardiac hypertrophy. Furthermore, the presence of severe secondary hyperparathyroidism (CRF + HP) showed not only an increase in interstitial cardiac fibrosis but also in molecular markers of fibrosis. Although moderate secondary hyperparathyroidism (CRF + NP) did not significantly increase interstitial cardiac fibrosis, it did increase some of the molecular markers of fibrosis. The results of this study suggest that part of these effects could be attributed to the increase in the degree of inflammation. Parathyroidectomy was able to attenuate cardiac hypertrophy and fibrosis and molecular markers of inflammation.
In a model similar to ours, no increase in myocardial hypertrophy, quantified as a relationship between heart weight and body weight, was observed in parathyroidectomized rats with a high phosphorus diet (CRF + PTx + rPTH + HP).12 However, other more sensitive parameters than the weight of the heart corrected for body weight are analyzed, such as the diameter of the cardiomyocytes used in the analysis of our study, parathyroidectomy did reduce cardiac hypertrophy, a finding that would indicate that the determination of cardiac hypertrophy by studying the corrected heart weight for body weight ratio is unspecific. Other authors suggest that as a correction factor, the relationship between heart weight and tibia length is preferable if there is a coexistence of body weight loss.22 A secondary analysis using the Mann-Whitney U statistical test showed that as compared with SHAM rats the development of moderate secondary hyperparathyroidism (CRF + NP) significantly increased several parameters of cardiac hypertrophy (heart weight referred to body weight, P = .001; septum thickness, P = .001; cardiomyocyte diameter, P = .005)
We cannot rule out that other factors contribute in addition to secondary hyperparathyroidism to the development of cardiac hypertrophy. In fact, FGF23 has shown its isolated and independent effect on the development of cardiac hypertrophy.23 In our study we observed an effect of FGF23 on the increase in the thickness of the septum. In addition, the high intact PTH is associated not only with increase in the thickness of the septum but it was also associated with greater diameter of cardiomyocytes.
There are no data in the literature linking the increase in cardiac fibrosis observed in the presence of elevated PTH levels with increases in systemic inflammation. In the present study, it was found that parathyroidectomy, both in rats on a normal phosphate diet or on a diet high in phosphate, attenuated the gene expression of TNF-α and Adam17. This findings suggest that these inflammation mediators are involved in the fibrosis process promoted by the presence of elevated PTH.24 In fact, this study shows that in the presence of chronic renal failure, the reduction in PTH, both with or without hyperphosphatemia, would play an important role in reducing interstitial cardiac fibrosis observed in CKD, a fact observed by other authors6,7 which indicate that the development of secondary hyperparathyroidism is an important risk factor for the development of myocardial fibrosis and that this could be reversed with parathyroidectomy.25
Analysis of the gene expression of fibrosis-related molecular markers supports the important role that increased PTH plays in the genesis of cardiac fibrosis. Comparison of the two groups of animals fed a high phosphorus diet (CRF + HP vs CRF + PTx + rPTH + HP) shows that in first group, there was a significant increase in the gene expression of type 1 collagen and of fibronectin, two of the most representative proteins of the extracellular matrix. The TGF-β1 was also increased probably reflecting what was observed at the level of interstitial cardiac fibrosis with Masson's trichrome staining. Other authors, analyzed some proteins involved in cardiac remodeling in a parathyroidectomized rat model and they found that the protein expression of TGF-β is also highly influenced by the levels of PTH.26 The postulated hypothesis has been that protein kinase C and other proteins, such as TGF-β1, would be activated through PTH, which in turn would stimulate fibroblast proliferation, collagen synthesis and fibrosis, favoring cardiac fibrosis.27 Of the molecular markers studied, CTGF was the only one that did not follow the same pattern and the parathyroidectomy, although it showed a trend (P = .09), did not attenuate its increase in the group of animals fed a high phosphorus diet. These results support the important role that secondary hyperparathyroidism would have in the development of cardiac hypertrophy and fibrosis.26
However, despite the possible effect that high intact PTH levels may have on the development of cardiac fibrosis, we cannot rule out other factors such as FGF23, which is very high in the CRF + HP group, as a consequence of the high phosphorus content in the diet. The lower degree of cardiac fibrosis observed in the parathyroidectomized group with a high phosphorus diet could be attributable to the drastic decrease in intact PTH, but also to the lower level of FGF23 achieved in this group.
This effect was observed with greater intensity with the stimulation of a diet rich in phosphorus, but it was also observed in those animals fed a normal diet (group 1), in which parathyroidectomy (CRF + PTX + NP) attenuated the increases of some of the molecular markers of fibrosis observed in the non-parathyroidectomized group (CRF + NP), a group that showed a clear increase in interstitial cardiac fibrosis compared to the SHAM group (P = .002, results not shown). Unlike the possible implication of PTH, and also of FGF23, in the development of cardiac fibrosis in animals with a high phosphorus diet, in the case of animals on a normal phosphorus diet, less cardiac fibrosis in some of the molecular markers cannot be attributed to the high FGF23 where the levels of the parathyroidectomized group were even higher. In this case it seems clear that PTH is the determining factor.
The confirmation of the role of PTH as the main determinant of the development of cardiac fibrosis seems to be corroborated by the important association observed between intact PTH levels and cardiac fibrosis, both at the histological level and with the rest of the molecular markers analyzed. On the contrary, FGF23 did not show associations with any of the parameters used to quantify cardiac fibrosis.
It is postulated that part of the effect that an increase in PTH has on the genesis of cardiac fibrosis would be due to increases in aldosterone that could be attenuated or prevented with parathyroidectomy, probably through avoiding an increase in intracellular calcium promoted by elevations in PTH.14 This important effect of calcium-mediated PTH is supported by the results of our study in which it was observed that the gene expression of the calcium channel TCRP6 at the cardiac level was attenuated with parathyroidectomy, decreasing more than 60%, both in the group with a normal phosphorus diet (P = .036) as in rats on a high phosphorus diet (P = .010) (data not shown). This suggests that at the least part of the cardiac complications could be mediated by a direct effect of PTH on the activation of calcium channels with secondary mobilization of this calcium from the sarcoplasmic reticulum to the cytoplasm of thecardiomyocyte.28
As possible limitations of this study, we can highlight that despite using a replacement dose of PTH 1-34, similar to that described by other authors to achieve physiological replacement in parathyroidectomized groups,16 this was not fully achieved as shown by the Observed hypocalcemia that was more marked in animals with a diet high in phosphorus, a fact that has also been shown by other authors using a similar dose of PTH 1-34.12
Another possible limitation is that we could not rule out that other factors, in addition to hyperparathyroidism, could contribute to the development of cardiac hypertrophy and fibrosis, such as FGF23, hypocalcemia, or calcitriol deficiency. In fact, there are authors who find a negative and independent effect of FGF23 on the development of cardiac fibrosis.29,30 However, the absence of correlations of FGF23 and calcitriol with cardiac fibrosis and molecular markers of fibrosis and the important correlation between intact PTH and cardiac fibrosis markers indicates the prominent role of secondary hyperparathyroidism in the development of cardiac fibrosis on top of the other factors mentioned above.
In summary, the present study confirms the important effect that hyperphosphatemia has on the genesis of secondary hyperparathyroidism and kidney damage manifested by a significant drop in creatinine clearance in rats with elevated PTH, a reduction in renal function that is independent of PTH levels. The study also shows that the parameters of inflammation, fibrosis, and growth of cardiomyocytes are fundamentally related to PTH levels, since in the presence of similar severe hyperphosphatemia, parathyroidectomy reduced the values of inflammatory parameters and cardiac hypertrophy and fibrosis.
FinancingThis work has been possible thanks to a grant to nephrology research in 2016 on “Search for new therapies that reduce the morbidity and mortality associated with chronic kidney disease.” This study has also been funded by the Carlos III Health Institute (ISCIII) - Health Research Fund (PI 07/0893, PI 10/00896, PI 13/00014, PI 16/00637, PI 17/00715 and PI19/00532), European Regional Development Fund (ERDF), Science, Technology and Innovation Plan 2013–2017 and 2018–2022 of the Principality of Asturias (GRUPIN14-028, IDI-2018-000152), Fundación Renal Íñigo Álvarez de Toledo (FRIAT) and Retic REDinREN of ISCIII (RD06/0016/1013, RD12/0021/0023, RD16/0009/0017, RD12/0021/0006 and RD16/0009/0018). Laura Martinez-Arias has been funded by ISCIII-FINBA (PI 16/00637 and PI 17/00384); Sara Panizo-García for FINBA-REDinREN and IDI-2018-000152; Natalia Carrillo-López for GRUPIN14-028 and IDI-2018-000152; Sara Fernández-Villabrille by IDI-2018-000152 and PI 17/00715; Julia Martín-Vírgala for a scholarship from the University of Oviedo and Beatriz Martín-Carro for ISCIII-FINBA (PI17/00384) and by a Severo Ochoa scholarship, program BP19-057, from the Principality of Asturias.
Authorship/collaboratorsLMA and SPG had the same contribution as first authors; NCL and MND as last signatories; and JBCA and MND as corresponding authors.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Martínez-Arias L, Panizo-García S, Martín-Vírgala J, Martín-Carro B, Fernández-Villabrille S, Avello-Llano N, et al. Contribución de fósforo y PTH al desarrollo de hipertrofia y fibrosis cardiaca en un modelo experimental de insuficiencia renal crónica. Nefrologia. 2022;41:640–651.