The clinical scenarios in which there is more evidence of the adverse effects of congestion in terms of mortality and hospital admissions are acute heart failure (HF) and the critically ill patient.1,2 However, in nephrology, the scope of this concept is extended to a wide range of both acute and chronic clinical conditions. The concept of congestion has evolved from the earliest descriptions by the Egyptians, throught the definition proposed by Starling to explain oedema formation,3 the understanding of the systemic effects of congestion in different organs.4 A clear example of the conceptual evolution of congestion is cardiorenal syndrome type 1 (CRS1) in which we assumed that acute kidney injury was associated with a hypoperfusion-related mechanism of damage (by an anterograde mechanism). However, more than 60% of patients with acute HF have congestion without hypoperfusion.5 In these patients, increased central venous pressure is transmitted through the renal veins (low-resistance vessels), increasing renal afterload and intrarenal pressure. The increase in pressure reduces Kidney perfusion and intratubular flow. This leads to a decrease in glomerular filtration rate and an increase in sodium and water retention, mediated by activation of the renin-angiotensin aldosterone system; together with tubular damage mediated by activation of pro-inflammatory mechanisms, among others (congestive nephropathy).6 We also know that patients with congestion have lower survival, longer hospital stays, and higher readmission rates.1,7
Adequate diagnosis of congestion is a challenge for the clinician. Although the presence of classic symptoms and signs such as dyspnea, orthopnea, jugular ingurgitation, oedema, and crackles are helpful, their sensitivity is limited and in many cases complex.8 An example of these limitations is hyponatremia, where assessment of extracellular volume is an immediate therapeutic approach. In this scenario, Chung et al. correctly identified only 47% of hypovolaemic patients and 48% of euvolaemic patients using classical clinical parameters.9 This limitation is particularly evident when distinguishing between vascular congestion and/or tissue congestion, which is key to therapeutic interventions aimed at enhancing increased natriuresis or fluid redistribution.10 In the 1990s, changes in portal vein flow assessed by pulsed Doppler (PD) in HF were described.11 Since then, bedside ultrasound or "Point-of-Care UltraSonography" (PoCUS) has become a useful tool to complement the physical examination of the patient with congestion.12 PoCUS answers a specific question non-invasively, in real time and reproducibly, allowing targeted therapeutic intervention. This is a different objective from that of a standard radiological or cardiological assessment.13
The assessment of congestion in the nephrology patient using PoCUS has three strategies. Lung UltraSound (LUS), which provides a rapid and accurate assessment of tissue congestion. "Venus Excess Ultrasound Grading System" (VExUS), which assesses vascular congestion using venous PD to identify and grade congestion, and the examination of cardiac and valvular morphology and function using echocardiography or "Focused Cardiac UltraSound" (FoCUS).
Ultrasound assessment of congestion begins with tissue assessment using LUS, noting the presence of B-lines or "pleural comets" in 8 anterior chest projections. "Pleural comets" are vertical, hyperechoic artefacts of the pleura that translate into interstitial changes associated with the presence of transudate or exudate.14 The presence of 3 or more B-lines in2 or more planes is associated with tissue congestion of the lung parenchyma,15 and this assessment also allows detection of the presence of pleural effusion.16 LUS has been shown to be more sensitive than chest radiography and physical examination in the detecting acute pulmonary oedema in patients with acute HF and small pleural effusions.17 On the other hand, although the estimation of left ventricular (LV) filling pressures are estimated by conventional echocardiography and require experience, a correlation between the presence of B lines by LUS and increased LV filling pressures has been reported.18 Even the presence of B lines in HF patients at hospital discharge has been associated with increased readmission and mortality rates.19 In a study conducted by Loutradis et al. in haemodialysis patients, the systematic use of LUS allowed a more accurate dry weight adjustment, contributing to a safe and effective blood pressure lowering.20 The LUST study (Lung water by ultraSound guided treatment to prevent death and cardiovascular complications in High Risk ESRD patients with cardiomyopathy Trial, presented at the 58th ERA-EDTA), demostrated that LUS as an effective strategy to safely guide ultrafiltration in hemodialysis patients, associated with fewer recurrences of decompensated HF and fewer episodes of intra-dialysis hypotension. Furthermore, LUS-guided ultrafiltration by LUS was associated with an accompanied by an improvement in LV systolic and diastolic function and a reduction in LV mass growth, although without achieving significant differences in the primary outcomes.21,22 Therefore, we consider LUS to be a useful tool in the assessment of pulmonary congestion, but with the need to complement it with the assessment of vascular congestion by VExUS. VExUS allows for the identification and stratification of vascular congestion by examining the inferior vena cava (IVC), suprahepatic veins (SHV), portal vein (VP) and intrarenal vessels (IRV).12,23
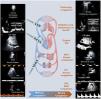
Under normal conditions there is no pulsatility in the venous capillaries and venules, but as the veins approach the heart they acquire pulsatility which is transmitted retrogradely by the pressure and volume oscillations of the right atrium (RA) and right ventricle (RV). Assessment of vascular congestion begins with examination of the IVC along its longitudinal axis 2 cm from its RA entrance. If the diameter is less than 2 cm, venous congestion is excluded, whereas if it is greater than 2 cm, the rest of the venous system needs to be assessed in order to assess and grade the organic compromise associated with the congestion. It can also be used to exclude dilatation of the IVC due to non-congestive causes. The PD of the VSH, branches of the IVC (pulsatile), allows the identification of the wave. The initial anterograde "a" wave of atrial contraction, the retrograde "S" wave of RV systole, which is larger than the "D" wave of RV diastole (Fig. 1). The changes in flow will determine the severity of the congestion. In mild-moderate congestion, the "S" wave is smaller in magnitude than the "D" wave (S < D) and in severe congestion the "S" wave changes to retrograde flow (flow towards the heart ).
On the left normal ultrasound pattern using LUS, FoCUS and VExUS. On the right severe congestion pattern.
bdRV: right ventricle basal diameter; TAPSE: Tricuspid Annular Plane Systolic Excursion; IVC: inferior vena cava; RV: right ventricle; PV: portal vein; IRV: intrarenal vessels, SHV: suprahepatic veins.
As mentioned above, the PV and IRV have no pulsatility due to their distance from the great vessels and under normal conditions the PD shows a continuous flow.24 In mild-moderate congestion the PV will change from a continuous flow to a pulsatile flow with a pulsatility index between 30–50% and in severe congestion the pulsatility will be greater than 50%. Finally, IRV assessment allows the identification of kidney involvement. In mild to moderate congestion, a biphasic flow is observed with the appearance of two systolic «S» and diastolic «D» waves, and in severe congestion, a monophasic flow will be observed with a single «D» wave during the cardiac cycle (Fig. 1). The IRV study with PD also allows calculation of the resistivity index (RI), which in the congestion scenario may be altered and result in increased intrarenal resistance. However, other situations such as atherosclerosis, parenchymal lesions, among others, may alter them.6
The visualization of the size of the IVC and the PD of the described venous territories are integrated into a congestion severity score (VExUS score) in which grade 0 has an IVC < 2 cm, grade 1 has an IVC ≥ 2 cm and PD with normal or mildly altered patterns, grade 2 has IVC ≥ 2 cm, with at least one PD severity pattern and grade 3 has IVC ≥ 2 cm, with two or more PD severity patterns23 (Table 1). VExUS has been validated mainly in SCR1 and critically ill patients such that, in SCR1, IRV flow study using PD showed a better correlation with congestion than RI, and correlated with increased RA pressure measured by right catheterization and was associated with worse outcomes in congested patients compared with those with mild congestion or no congestion.24 On the other hand, deplection depletive therapy by VExUS in the critically ill patient was significantly correlated with kidney recovery in those patients with acute kidney injury.25
Proposed classification for global assessment of congestion according to LUS and VExUS. Morphological and functional impairments of the RV according to FoCUS.
| Absence of congestion | Mild–moderate congestion | Severe congestion | |
|---|---|---|---|
| Lung ultrasound (LUS) | Absence of B lines | More than 3 B lines | More than 3 B lines |
| VExUS | |||
| IVC | <2 cm | ≥2 cm | ≥2 cm |
| SHV | Pattern S > D | Pattern S < D | Reverse S wave |
| PV | Continuous flow | Pulsatility 30−49% | Pulsatility >50% |
| IRV | Continuous flow | Biphasic flow | Monophasic flow |
| SD pattern | D pattern | ||
| Echocardioscopy (FoCUS) | RV dilation: bdRV > 41 mm28 | ||
| RV dysfunction: TAPSE < 17 mm28 | |||
Focus: focus cardiac ultrasound; LUS: lung ultrasound; IVC: inferior vena cava; RV: right ventricle; VExUS: venous excess ultrasound grading system; PV: portal vein; IRV: intrarenal vessels; SHV: suprahepatic veins.
Adapted from: Beaubien-Souligny et al. 23
The use of VExUS is not without confounding factors, as changes in PD patterns without congestion may be observed, for example in patients with lower muscle mass index, liver parenchymal abnormalities, severe tricuspid regurgitation or advanced chronic kidney disease.26
Right HF is characterized by the inability of the RV to generate adequate stroke volume, leading to the development of venous congestion. This relationship makes the correlation of venous congestion with cardiac assessment critical. FoCUS is the strategy that allows for morphological and functional assessment of the RV in different classical echocardiographic planes.27 By comparing the size of the RV with the LV and assessing septal motion, changes in RV volume and pressure can be described. In the same classic planes, LV and RV systolic function can be assessed relatively easily by direct visualization or by using tools to estimate it.28,29 An indirect, widely used and relatively simple method of quantifying RV function is the M-mode measurement of Tricuspid Annular Plane Systolic Excursion (TAPSE).28 FoCUS also allows rapid assessment of the presence of pericardial effusion and valvular abnormalities such as tricuspid regurgitation.14,29
Most patients with acute or chronic kidney disease have impaired volume management. PoCUS allows the assessment of tissular and vascular congestion, the rapid, dynamic and reproducible personalisation of decongestive therapy in any clinical scenario and the correlation of these findings with FoCUS.
Beyond the clinical applicability of PoCUS, more recent literature has described the correlation between PD parameters and biomarkers of congestion and myocardial damage. A positive linear correlation has been described between PD congestion patterns and elevation of novel congestion biomarkers such as carbohydrate antigen 125 (CA125).30 Another diagnostic tool widely used in nephrology is electrical bioimpedance, which allows estimation of body composition. The knowledge of extracellular water and the estimation of dry weight using electrical bioimpedance could help to improve haemodynamic tolerance to haemodialysis.31 The combination of PoCUS results with biomarkers (a concept described as “bio-sono markers”) and electrical bioimpedance parameters will allow predictive models that include classical clinical variables with the aim of improving the ability to predict clinical outcomes.
The integration of these strategies requires regular training by the nephrologist and should be considered for inclusion in the training of nephrology residents in the next decade.16 The ability to interpret these strategies in a routine and reproducible methodology allows a more accurate approximation of the congestion scenario, as well as taking into account the fundamental importance of the right heart. In conclusion, a path of no return for clinical assessment and personalized diagnostic guidance is opening up before our eyes, as well as a door to new avenues of research that represent one of the most exciting challenges in cardiorenal medicine, a challenge for nephrology in the coming decade.