To assess the effects of pharmacological interventions in patients with idiopathic hypercalciuria.
MethodsWe performed a search of multiple databases, trial registries, grey literature and conference proceedings up to October 2019. We included randomized and quasi-randomized controlled trials that examined any pharmacological intervention for preventing complications of idiopathic hypercalciuria (given for at least four months and six of follow-up). The primary outcomes were stone-free patients, urinary symptoms and severe adverse events.
ResultsWe included five RCTs (n=446 patients, all adults, 4 in individuals with kidney stones and 1 in postmenopausal women with osteoporosis). Diuretics were likely to increase the number of stone-free patients (RR 1.61, 95% CI 1.33–1.96, moderate quality of evidence (QoE)); 274 more stone-free patients/1000 patients treated (95% CI: 148–432) and produced a slight decrease in the stone formation rate (mean difference −0.18, 95% CI −0.30 to −0.06, low QoE); 180 fewer stones/year/1000 patients treated (95% CI: 300 r to 60). No data on urinary symptoms were reported. The association between diuretic use and severe adverse events was uncertain (RR 5.00, 95% CI 0.60–41.88, very low QoE); 4 more severe adverse events/1000 patients treated (95% CI: 0 fewer to 39 more).
ConclusionsThe addition of diuretics to a normal or modified diet probably reduces the number of stone recurrences and may decrease the stone formation rate. It is uncertain whether diuretics increase the occurrence of severe adverse events. There were no studies investigating other outcomes or in children.
Evaluar los efectos de intervenciones farmacológicas en pacientes con hipercalciuria idiopática.
MétodosRealizamos una búsqueda en múltiples bases de datos, registros de ensayos, literatura gris y actas de congresos hasta octubre de 2019. Incluimos ensayos clínicos aleatorizados y cuasialeatorizados que examinaban cualquier intervención farmacológica para prevenir las complicaciones de la hipercalciuria idiopática (mínimo 4 meses de intervención y 6 meses de seguimiento). Los outcomes primarios fueron pacientes libres de cálculos, síntomas urinarios y efectos adversos graves.
ResultadosIncluimos 5 RCT (n=446 pacientes, todos adultos, 4 en individuos con cálculos renales y uno en mujeres posmenopáusicas con osteoporosis). Los diuréticos aumentaban probablemente el número de pacientes libres de cálculos (RR 1,61; IC 95%: 1,33 a 1,96, moderada calidad de evidencia [QoE]); 274 más pacientes libres de cálculos/1.000 pacientes tratados (IC 95%: 148 a 432) y producían una ligera disminución en la tasa de formación de cálculos (diferencia media −0,18; IC 95%: −0,30 a −0,06, baja QoE); 180 menos cálculos/año/1.000 pacientes tratados (IC 95%: 300 a 60). No se informaron datos sobre síntomas urinarios. La asociación entre el uso de diuréticos y los efectos adversos graves fue incierta (RR 5,00; IC 95%: 0,60 a 41,88, muy baja QoE); 4 efectos adversos severos más/1.000 pacientes tratados (IC 95%: 0 a 39).
ConclusionesLos diuréticos añadidos a una dieta normal o modificada probablemente reducen la aparición de cálculos y pueden disminuir su tasa de formación. Es incierto si los diuréticos incrementan la ocurrencia de efectos adversos graves. No se encontraron estudios que investigaran otros outcomes o realizados en niños.
Idiopathic hypercalciuria (IH), one of the most common hereditary metabolic anomalies, is defined as calcium excretion greater than 300mg/24h in men and 250mg/day in women; it is also defined as Ca urine>4mg/kg (body weight) or Ca/Creatinine>0.20mg/mg among patients of both sexes with an unrestricted calcium diet and no evidence of secondary causes.1 Prevalence rates ranging from 2.9% to 22% have been reported in the healthy population.2,3
The pathophysiology of IH is highly complex. There are three main pathophysiological mechanisms: reduced tubular reabsorption, increased intestinal absorption and increased bone resorption. However, there are several interrelationships between all these mechanisms as well as with diet factors and most patients manifest different features belonging to more than one mechanism.4 As an example of this interrelationships, there is evidence that one IH development could be related with an increase of vitamin D receptors (observed in the gut and bone of IH rats as well as in monocytes from both rats and humans) and/or a raise in the functional activity of the calcitriol-VDR complexes that induced an increase in both intestinal calcium absorption and bone resorption.5
One of the undesired consequences of IH is stone formation that starts when concentrations of calcium and oxalate reach saturation. Approximately 80% of all kidney stones contain calcium, and at least 40–60% of all patients who form calcium stones are found to have hypercalciuria when tested.6 Therefore, hypercalciuria contributes to kidney stone disease in adults and children.7
The reported prevalence of renal stones in different countries and over time is highly variable, ranging from 1.7% to 18.5%,8 and recurrence is approximately 50%.9 Diverse morbidity manifestations such as obstruction, haematuria, frequency-dysuria syndrome, abdominal and lumbar pain, and recurrent urinary infections10 are related to urolithiasis. The potentially most dangerous aspect of stone disease is the combination of obstruction and infection in the upper urinary tract.11
Moreover, there is also a relationship between hypercalciuria and osteopenia and osteoporosis.12–15 Up to 22% of children with IH have osteopenia,16 the long-term seriousness of which has yet to be determined even though many children with osteopenia normalized their bone mineral density without any pharmacological treatment17. In relation to the therapeutic options for the treatment of IH and its comorbidities, numerous pharmacological treatments can decrease levels of calciuria or the urinary crystallization index. However, the efficacy of these treatments in controlling the illness and preventing the clinical manifestations of IH remains controversial. Several meta-analyses have evaluated the effect of medications on preventing kidney stones,18 although very few have focused on patients with proven hypercalciuria.
Thiazides increase calcium reabsorption in the distal tubule and have demonstrated efficacy in preventing recurrent calcium nephrolithiasis; however, they can also cause glucose intolerance, dyslipidaemia, hyperuricaemia and hypokalaemia, which in turn leads to intracellular metabolic acidosis and hypocitraturia.19 Thiazides may improve bone mass density (BMD) in patients with recurrent stones and hypercalciuria, although the long-term effect of their use is still not very clear.20–22 Indapamide, a non-thiazide diuretic, seems to have similar effects and safety profiles.23
Other drugs, such as citrate salts, orthophosphates or bisphosphonates, have also shown some beneficial effects, but they are not free of potential side effects.24,25
There are several recent studies and reviews on the prevention of recurrent kidney stones,26–37 but these studies do not analyze the effects of pharmacological interventions in the subgroup of individuals with IH. In 2009, our group published a systematic review about the pharmacological treatment of IH,38,39 and our present aim is to update that previous review while shifting the focus to patient-important outcomes associated with IH, such as stone formation, severe adverse events and urinary symptoms.
The objective of this systematic review was to assess how pharmacological interventions can influence several relevant outcomes in patients with IH.
Materials and methodsSearch strategy and selection criteriaIn October 2019, we performed a comprehensive search of the Cochrane Central Register of Controlled Trials, MEDLINE (Ovid), and Embase databases; clinical trials registries; and the grey literature. To identify any studies that may have been missed during the literature search, we checked the reference lists of eligible studies and contacted the authors of identified studies for knowledge of any published or unpublished studies, including new studies, additional studies, or works in progress. Two review authors independently screened all potentially relevant records and classified studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions.40 We included randomized and quasi-randomized controlled trials (RCTs and q-RCTs), regardless of their publication status or language of publication.
Types of participantsStudies performed on adults or children with IH, defined as calcium excretion greater than 4mg/kg/day, 250mg/day in women or 300mg/day in men (in the absence of any evidence of secondary cause) undergoing pharmacological treatment to control the illness and its complications were included. We excluded studies that enrolled patients with secondary hypercalciuria or patients suffering other illnesses that could cause osteopenia or urinary stones.
Types of interventionsWe investigated the effects of any pharmacological intervention (longer than 4 months) for preventing complications in IH versus a control/comparator (placebo, other pharmacological intervention or a different administration mode or dose of the same treatment). We assessed only those interventions with a follow-up period of at least six months.
Types of outcome measuresThe primary outcomes of the review were stone-free patients, urinary symptoms and severe adverse events. The secondary outcomes included the stone formation rate, changes in BMD, quality of life, calciuria and any adverse events. We include a ‘summary of findings’ table reporting the primary outcomes and stone formation rate using GRADEpro.41
Data collection and analysisFour review authors working in pairs assessed all studies using a data extraction form and followed the domain-based risk of bias evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions.40 We attempted to obtain numbers of events and totals for the population for dichotomous outcomes and means with standard deviations or data necessary to calculate these statistics for continuous outcomes. Unless there was good evidence for homogeneous effects across studies, we summarized the data using a random-effects model. We interpreted the random-effects meta-analyses with due consideration of the whole distribution of effects. We assessed heterogeneity statistically with the I2 value; I2>50% indicated substantial heterogeneity.42 We planned to test for asymmetry using the funnel plot, but the number of trials was insufficient for comparison. We used Review Manager 5 software (Cochrane Collaboration, Copenhagen, Denmark) to perform the statistical analyses. When possible, we explored the effect of bias in the effect estimates and performed predefined subgroup analysis.
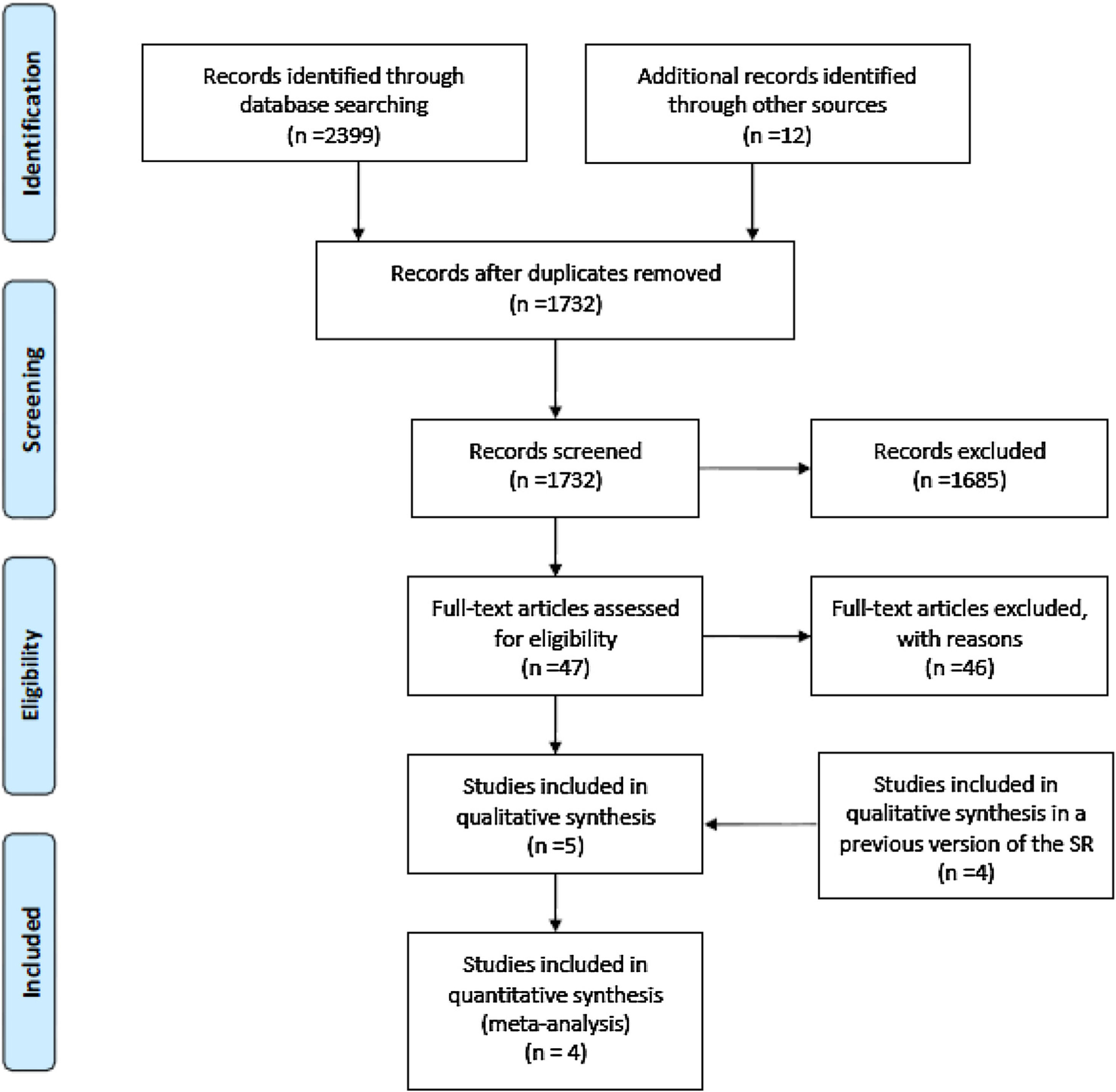
ResultsFig. 1 shows the study flow diagram. Detailed descriptions of the included and excluded studies are shown in Table 1 and Additional file 1, respectively.
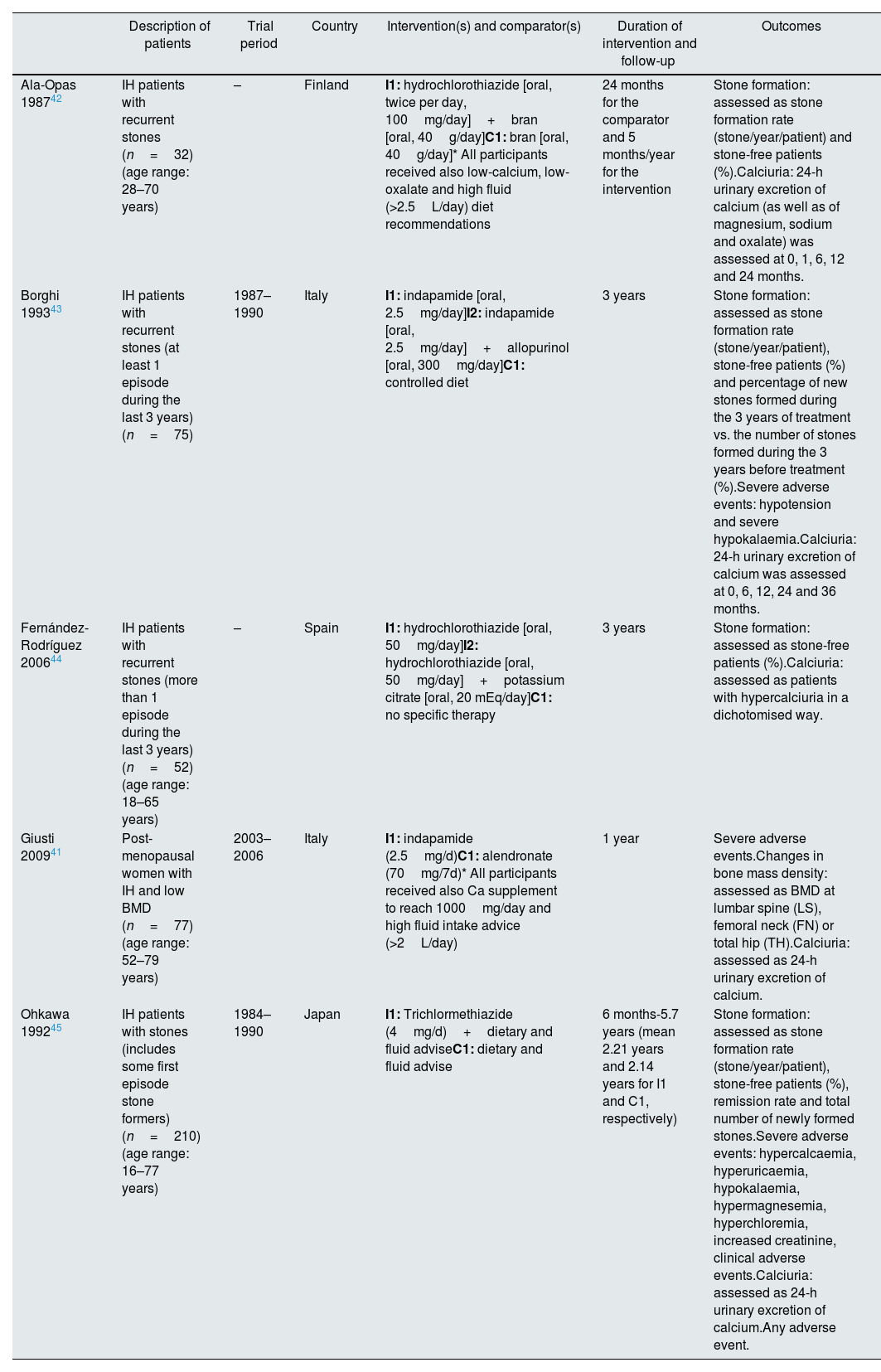
Baseline characteristics and description of the interventions in the included studies.
| Description of patients | Trial period | Country | Intervention(s) and comparator(s) | Duration of intervention and follow-up | Outcomes | |
|---|---|---|---|---|---|---|
| Ala-Opas 198742 | IH patients with recurrent stones (n=32) (age range: 28–70 years) | – | Finland | I1: hydrochlorothiazide [oral, twice per day, 100mg/day]+bran [oral, 40g/day]C1: bran [oral, 40g/day]* All participants received also low-calcium, low-oxalate and high fluid (>2.5L/day) diet recommendations | 24 months for the comparator and 5 months/year for the intervention | Stone formation: assessed as stone formation rate (stone/year/patient) and stone-free patients (%).Calciuria: 24-h urinary excretion of calcium (as well as of magnesium, sodium and oxalate) was assessed at 0, 1, 6, 12 and 24 months. |
| Borghi 199343 | IH patients with recurrent stones (at least 1 episode during the last 3 years) (n=75) | 1987–1990 | Italy | I1: indapamide [oral, 2.5mg/day]I2: indapamide [oral, 2.5mg/day]+allopurinol [oral, 300mg/day]C1: controlled diet | 3 years | Stone formation: assessed as stone formation rate (stone/year/patient), stone-free patients (%) and percentage of new stones formed during the 3 years of treatment vs. the number of stones formed during the 3 years before treatment (%).Severe adverse events: hypotension and severe hypokalaemia.Calciuria: 24-h urinary excretion of calcium was assessed at 0, 6, 12, 24 and 36 months. |
| Fernández-Rodríguez 200644 | IH patients with recurrent stones (more than 1 episode during the last 3 years) (n=52) (age range: 18–65 years) | – | Spain | I1: hydrochlorothiazide [oral, 50mg/day]I2: hydrochlorothiazide [oral, 50mg/day]+potassium citrate [oral, 20 mEq/day]C1: no specific therapy | 3 years | Stone formation: assessed as stone-free patients (%).Calciuria: assessed as patients with hypercalciuria in a dichotomised way. |
| Giusti 200941 | Post-menopausal women with IH and low BMD (n=77) (age range: 52–79 years) | 2003–2006 | Italy | I1: indapamide (2.5mg/d)C1: alendronate (70mg/7d)* All participants received also Ca supplement to reach 1000mg/day and high fluid intake advice (>2L/day) | 1 year | Severe adverse events.Changes in bone mass density: assessed as BMD at lumbar spine (LS), femoral neck (FN) or total hip (TH).Calciuria: assessed as 24-h urinary excretion of calcium. |
| Ohkawa 199245 | IH patients with stones (includes some first episode stone formers) (n=210) (age range: 16–77 years) | 1984–1990 | Japan | I1: Trichlormethiazide (4mg/d)+dietary and fluid adviseC1: dietary and fluid advise | 6 months-5.7 years (mean 2.21 years and 2.14 years for I1 and C1, respectively) | Stone formation: assessed as stone formation rate (stone/year/patient), stone-free patients (%), remission rate and total number of newly formed stones.Severe adverse events: hypercalcaemia, hyperuricaemia, hypokalaemia, hypermagnesemia, hyperchloremia, increased creatinine, clinical adverse events.Calciuria: assessed as 24-h urinary excretion of calcium.Any adverse event. |
Included studies are summarized in Table 1. Based on the results of the new searches, we included a new study, which was conducted by Giusti et al.43 Among the five studies included in the previous version of the systematic review, we included four of them (studies conducted by Ala-Opas et al.,44 Borghi et al.,45 Fernández-Rodríguez et al.,46 and Ohkawa et al.47) in the current analysis, whereas the fifth (conducted by Breslau et al.48) was deemed ineligible after reassessment because of the short treatment and follow-up period. All five included studies were randomized controlled trials set in ambulatory care or academic facilities, with sample sizes that ranged from 32 to 210 patients. The studies included a total of 586 randomized patients, of which 446 had IH and were analyzed in this review. Four of the studies44–47 included patients with urinary stones, and one study43 included postmenopausal patients in which hypercalciuria was detected in the context of a BMD evaluation. All studies included the assessment of diuretics in one of the treatment arms. One study43 compared the use of diuretics with alendronate (a medication used for the treatment of BMD disorders). The outcomes were assessed at the end of the intervention in four studies43,45–47), while in the fifth,44 a longer period of follow-up was established.
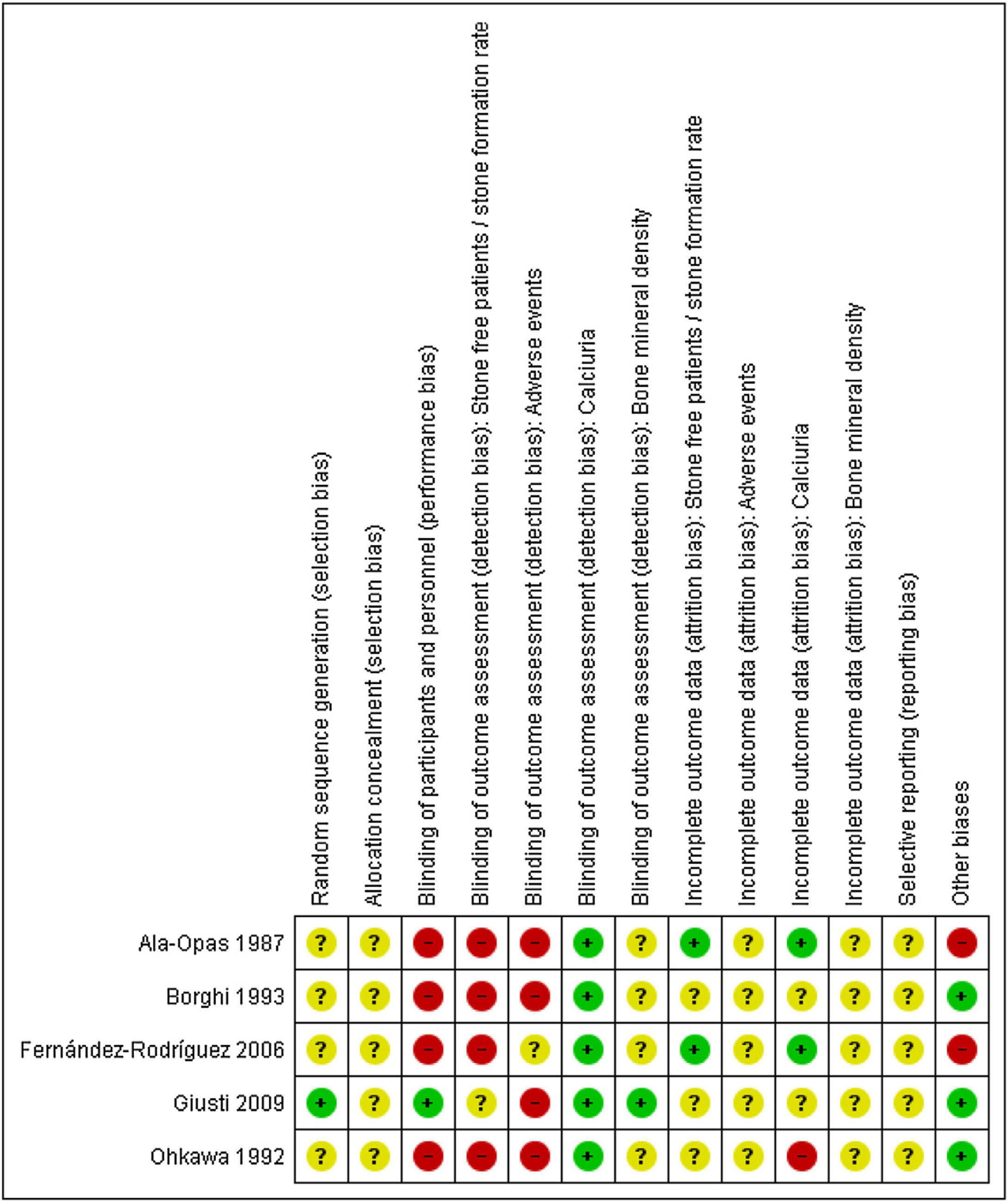
The results from the risk of bias assessments are summarized in Fig. 2, and detailed judgement of each study can be found in Additional file 1. Most studies had poor reporting of their characteristics with an unclear risk of bias in multiple domains. All outcomes were judged to have a high risk of bias, mostly due to a lack of participant and personnel blinding.
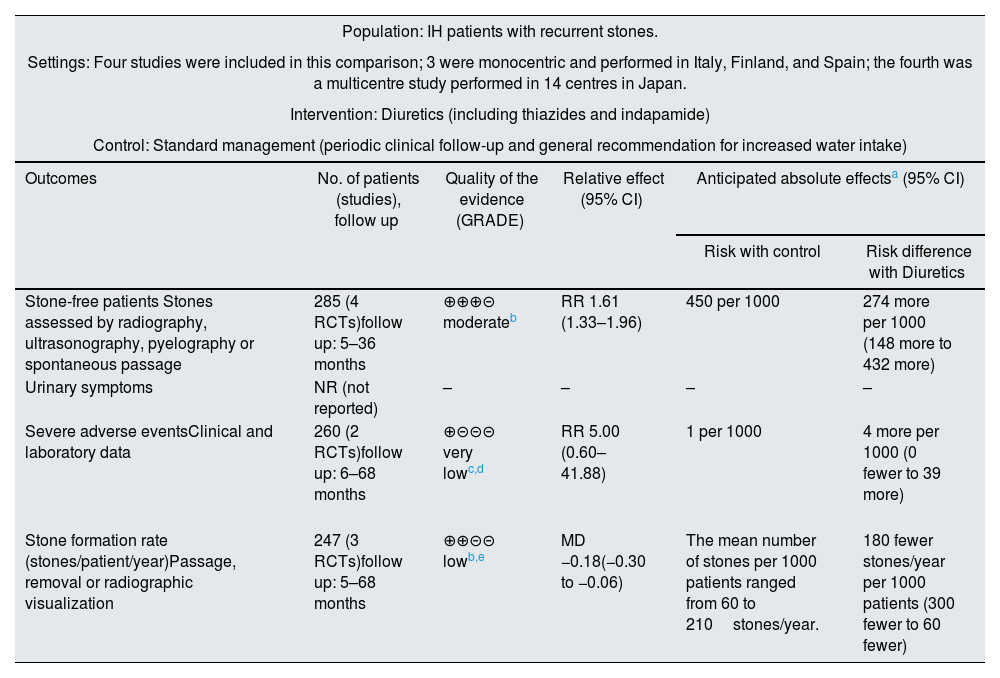
Effect of the intervention, Comparison 1: diuretics versus standard control of the disease or specific dietary recommendationsFour of the five included studies assessed the effect of the addition of diuretics to the standard control of the disease (periodic clinical follow-up and general recommendation for increasing water intake)45–47 or specific dietary recommendations.44 The results of this comparison are presented by outcome. None of the studies addressed urinary symptoms, changes in BMD or quality of life. The main outcomes are summarized in Table 2.
Summary of findings table for the comparison of diuretics versus control.
| Population: IH patients with recurrent stones. | |||||
|---|---|---|---|---|---|
| Settings: Four studies were included in this comparison; 3 were monocentric and performed in Italy, Finland, and Spain; the fourth was a multicentre study performed in 14 centres in Japan. | |||||
| Intervention: Diuretics (including thiazides and indapamide) | |||||
| Control: Standard management (periodic clinical follow-up and general recommendation for increased water intake) | |||||
| Outcomes | No. of patients (studies), follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effectsa (95% CI) | |
| Risk with control | Risk difference with Diuretics | ||||
| Stone-free patients Stones assessed by radiography, ultrasonography, pyelography or spontaneous passage | 285 (4 RCTs)follow up: 5–36 months | ⊕⊕⊕⊝ moderateb | RR 1.61 (1.33–1.96) | 450 per 1000 | 274 more per 1000 (148 more to 432 more) |
| Urinary symptoms | NR (not reported) | – | – | – | – |
| Severe adverse eventsClinical and laboratory data | 260 (2 RCTs)follow up: 6–68 months | ⊕⊝⊝⊝ very lowc,d | RR 5.00 (0.60–41.88) | 1 per 1000 | 4 more per 1000 (0 fewer to 39 more) |
| Stone formation rate (stones/patient/year)Passage, removal or radiographic visualization | 247 (3 RCTs)follow up: 5–68 months | ⊕⊕⊝⊝ lowb,e | MD −0.18(−0.30 to −0.06) | The mean number of stones per 1000 patients ranged from 60 to 210stones/year. | 180 fewer stones/year per 1000 patients (300 fewer to 60 fewer) |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). IH: Idiopathic hypercalciuria; CI: Confidence interval; RR: Risk ratio; OR: Odds ratio.
GRADE framework to evaluate the quality of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Downgraded by one level due to study limitations: a high risk of performance and detection bias, an unclear risk of selection bias and a high risk of other biases.
Downgraded by two levels due to severe imprecision: few events resulted in a wide confidence interval.
This outcome was reported in all four studies with a total of 285 patients.
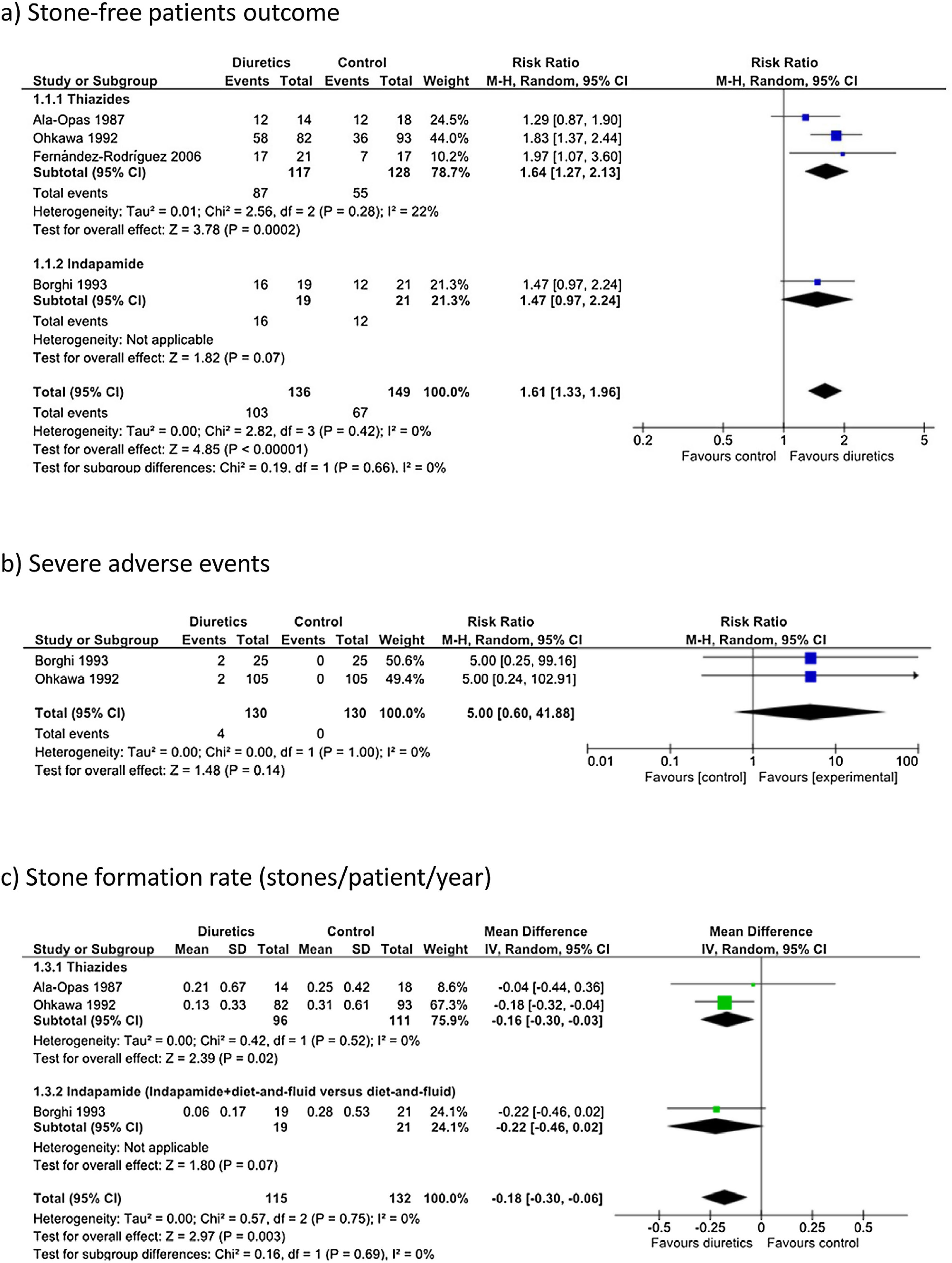
Diuretics likely increased the proportion of stone-free patients (Fig. 3a: RR 1.61, 95% CI 1.33–1.96). Assuming that the baseline probability of being stone-free is 45% (based on the observed average risk in the control groups of the analyzed studies), the administration of diuretics would result in 274 more stone-free individuals per 1000 patients treated (95% CI 148–432). Among patients treated with diuretics, 103 out of 136 (75.7%) were stone-free at the end of the follow-up, compared with 67 out of 149 (44.9%) in the control group. Although the follow-up periods were not homogeneous, particularly in the study conducted by Ohkawa et al.,47 there was no statistically significant heterogeneity (I2=0%). The QoE for this outcome was moderate due to the following study limitations: a high risk of performance and detection bias, an unclear risk of selection bias and a high risk of other biases.
Severe adverse eventsBorgi et al.,45 reported serious adverse events in two out of 25 patients treated with indapamide: one hypokalaemia event with serum potassium 2.6mEq/L and one severe hypotension event. In the study conducted by Ohkawa et al.,47 two out of 105 patients treated with thiazides had to stop treatment because of adverse events compared to no patients in the control group. In the studies conducted by Ala-Opas et al.,44 and Fernandez-Rodriguez et al.,46 there were no withdrawals due to adverse events, and no further information was provided regarding this outcome.
Overall, the use of diuretics may not cause severe adverse events when compared to placebo or no intervention (Fig. 3b: RR 5.00, 95% CI 0.60–41.88). Assuming that the baseline probability of severe adverse events is 0.1%, the administration of diuretics would result in 4 more cases per 1000 patients treated (95% CI 0 fewer to 39 more). The QoE was very low, and it was downgraded due to the following study limitations: a high risk of performance and detection bias, an unclear risk of selection bias, a high risk of other biases, and imprecision (few events, wide confidence interval).
Stone formation rate (secondary outcome)Three out of the four abovementioned studies (247 patients) reported stone formation rates.44,45,47 Diuretic treatment likely reduced the number of stones/patient/year (Fig. 3c: mean difference −0.18, 95% CI −0.30 to −0.06). Therefore, considering an average stone formation rate of 60–210stones/year in 1000 patients treated, the use of diuretics may cause the formation of 180 fewer stones/year (60–300fewer stones/year). The QoE was low, and it was downgraded due to the following study limitations: a high risk of performance and detection bias, an unclear risk of selection bias a high risk of other biases, and imprecision (wide confidence intervals that contained values above and below the thresholds for clinically meaningful effects).
Calciuria (secondary outcome)Fernandez-Rodriguez et al.,46 did not report calciuria but reported the number of patients with persistent hypercalciuria. At the 36-month follow-up, 15 of the 17 patients in the control group had persistent hypercalciuria, whereas 8 of the 21 patients who received diuretics had persistent hypercalciuria (RR 0.43, 95% CI 0.24–0.77).
Ohkawa et al.,47 reported that calciuria decreased to 0.567mmol/mol creatinine in the thiazide group and to 0.641mmol/mol in the control group at the one-month follow-up (no statistical testing between the groups was performed). Urinary calcium values were available in less than half of the patients at the six-month follow-up.
Borghi et al.,45 reported that calciuria decreased to 201mg/24h (SD 81) in the thiazide group and 330mg/24h (SD 97) in the control group at 36 months of follow-up (mean difference of −129.00, 95% CI −184.21 to −73.79).
Ala-Opas et al.,44 reported that calciuria at the 24-month follow-up was 0.34mmol/mol creatinine (SD 0.05) in the control group (bran only) and 0.30mmol/mol creatinine (SD 0.12) in the thiazide plus bran group (mean difference of −0.04mmol/mol creatinine 95% CI −0.11 to 0.03).
Using the random effects model for the meta-analysis, the results of these studies yielded a standardized mean difference (SMD) of −0.93 (95% CI −1.87 to 0.02; participants=72; studies=2; I2=72%).
The QoE for this outcome was very low due to an unclear or high risk of bias in most domains as well as inconsistency and imprecision (few patients in each group, wide confidence interval for the estimates).
Any adverse events (secondary outcome)In two of the studies, adverse events were not clearly reported. Ala-Opas et al.,44 stated that adverse events were “uncommon” in the thiazide+bran therapy group, and Fernández-Rodríguez et al.,46 provided no data on adverse effects.
In the study conducted by Ohkawa et al.,47 clinical adverse reactions, probably due to trichlormethiazide, were observed in nine patients (8.5%) in the thiazide group: dizziness in six, weakness in two and general malaise in one. Only three patients (3.7%) in the intervention group showed mild hyperuricaemia (0.50–0.51mmol/L) in an isolated check-up (versus no patients in the control group). Eight patients in the thiazide group (5.3%) presented with temporary hypokalaemia (2.8–3.4mmol/L) compared with no patients in the control group.
The QoE for this outcome was very low due to the high risk of performance and detection bias, the unclear risk in most other domains and imprecision (few events in each group).
Sensitivity analysesThe outcomes evaluated in this meta-analysis showed no statistical heterogeneity; therefore, we did not perform a sensitivity analysis to identify studies contributing to heterogeneity. Additionally, since heterogeneity was low, the estimates and confidence intervals were almost identical using fixed effects and random effects models. We could not perform a sensitivity analysis according to risk of bias since all study outcomes had a high risk of bias.
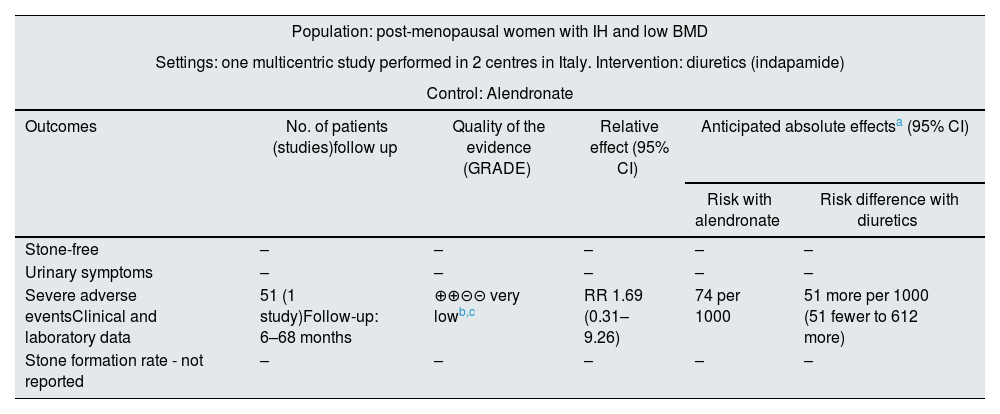
Effect of the interventions, Comparison 2: thiazide versus alendronateOnly one of the included studies43 assessed the effect of indapamide compared to alendronate. This study did not assess the effect of the intervention on stone formation, urinary symptoms, quality of life or non-severe adverse events. The main outcomes are summarized in Table 3.
Summary of findings for the comparison of diuretics versus alendronate.
| Population: post-menopausal women with IH and low BMD | |||||
|---|---|---|---|---|---|
| Settings: one multicentric study performed in 2 centres in Italy. Intervention: diuretics (indapamide) | |||||
| Control: Alendronate | |||||
| Outcomes | No. of patients (studies)follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effectsa (95% CI) | |
| Risk with alendronate | Risk difference with diuretics | ||||
| Stone-free | – | – | – | – | – |
| Urinary symptoms | – | – | – | – | – |
| Severe adverse eventsClinical and laboratory data | 51 (1 study)Follow-up: 6–68 months | ⊕⊕⊝⊝ very lowb,c | RR 1.69 (0.31–9.26) | 74 per 1000 | 51 more per 1000 (51 fewer to 612 more) |
| Stone formation rate - not reported | – | – | – | – | – |
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). IH: idiopathic hypercalciuria; CI: confidence interval; RR: risk ratio; OR: odds ratio.
GRADE framework to evaluate the quality of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Indapamide likely does not increase severe adverse events compared to alendronate (RR 1.69, 95% CI 0.31–9.26). The adverse events associated with alendronate treatment were gastrointestinal symptoms, and those associated with indapamide were severe hypotension. Assuming a 7.4% baseline probability of severe adverse events, the administration of diuretics would result in 51 more cases per 1000 patients treated (95% CI 51 fewer to 612 more).
The QoE for this outcome was low due to study limitations (a high risk of outcome assessment bias and an unclear risk of selection, attrition and other biases) and imprecision.
Changes in BMD (secondary outcome)Indapamide may not increase BMD (an increase of approximately 1.0±3.1% in the lumbar spine and a reduction of approximately 0.3±3.5% and 0.4±3.1% in femoral neck and total hip, respectively, were observed). In contrast, alendronate was able to induce an increase in BMD of approximately 5.8±4.2%, 3.9±7.9% and 2.0±3.6% in the lumbar spine, femoral neck and total hip, respectively. Patients treated with the combination of alendronate plus indapamide showed a greater increase in lumbar BMD compared with that observed in patients treated with alendronate alone: 8.2±5.3% and 5.8±4.2%, respectively (P<0.05).
The QoE for this outcome was low due to an unclear risk of bias in most domains and imprecision (small sample size, wide confidence intervals).
Calciuria (secondary outcome)Regarding 24-h calciuria values, Giusti et al.,43 showed decreased values after treatment compared with baseline in all groups, with reductions of 24.3±19.7% in the alendronate group and reductions of 34.7±18.2% in the indapamide group (P<0.001). The combination of indapamide and alendronate may cause a greater reduction in calciuria compared with alendronate or indapamide alone (reduction in 24-h calciuria levels up to 49.7±21%, P=0.012 versus indapamide alone and P<0.001 versus alendronate alone).
The QoE for this outcome was low due to an unclear risk of bias in most domains and imprecision (small sample size, wide confidence intervals).
DiscussionWe identified five RCTs that included a total of 446 hypercalciuric adult patients. We did not find studies in children.
The stone-free patient outcome was analyzed in four studies that included 285 patients and compared the effect of diuretics with standard control of the disease.43–47 The moderate to low QoE for these studies indicates that diuretics probably increase the number of stone-free patients and may reduce the stone formation rate when compared with controls.
We are uncertain whether the use of diuretics is associated with a greater incidence of severe adverse events, but among the 154 patients treated with diuretics, seven (4.5%) patients had to discontinue the treatment because of adverse events: four due to hypotension, two due to dizziness and general malaise and one due to severe hypokalaemia. None of these studies reported the effects of these interventions on urinary symptoms.
Overall, the QoE for studies of specific diuretic treatments is limited due to the use of different types of diuretics, variable doses, different follow-up periods, and variable imaging protocols.
The comparison of alendronate versus indapamide was addressed by one study with 77 participants.43 This study did not report the number of stone-free patients, urinary symptoms or stone formation rate. We are uncertain whether the use of diuretics is associated with a greater incidence of severe adverse events when compared with alendronate.
Reports based on small samples of patients suggested benefits of etidronate plus calcium supplementation49 or thiazide20,21 treatments for BMD in patients with hypercalciuria. Only Giusti 2009 assessed this outcome in hypercalciuric women with low BMD treated with indapamide and alendronate. The results after one year of treatment showed that indapamide had a negative effect on BMD compared with alendronate.
As part of the secondary outcomes, we analyzed the effects of pharmacological interventions on calciuria. Both diuretics and bisphosphonates showed an association with reduced calciuria, but we found some inconsistency across comparisons. In general, improvements were observed in the short term, but evidence for longer periods of control is insufficient. Two studies showed a considerable effect maintained for at least 36 months.45,46 However, these studies measured calciuria differently (one of them only dichotomously); therefore, the results should be interpreted with caution. Furthermore, calciuria is only one of the factors associated with stone formation and urinary symptom incidence; therefore, its reduction might not directly impact clinical outcomes.50
Although we conducted extensive and sensitive literature searches without language restriction, the possibility of publication bias remains. As four authors screened titles and abstracts of each record and extracted data independently, we assume that the risk of bias on the review process was low. We attempted to improve the number of studies included by contacting four authors for specific data for the hypercalciuric patients, but we were unsuccessful.
Several systematic reviews and guidelines have suggested that diuretics could act as a prophylactic treatment for patients with recurrent urolithiasis and a high risk of recurrence.36,51,52 Accordingly, our review showed that diuretic treatment in hypercalciuric patients with urolithiasis probably has beneficial effects; diuretic treatment was associated with an increased stone-free patient rate (RR 1.61, 95% CI 1.33–1.96) and a low incidence of severe adverse events (7/154 patients). To date, there is no evidence on the role of pharmacological interventions for preventing nephrolithiasis in individuals with hypercalciuria with no previous history of kidney stones.
A Cochrane review on dietary interventions for preventing complications in IH53 reported an increase in stone-free patients (RR 1.30, 95% CI 1.02–1.63) under a normal calcium, low protein and low salt diet versus a low calcium diet. The current review included studies comparing dietary intervention (low calcium diet) versus dietary plus diuretic treatment, which showed a higher reduction of risk with combined therapies (RR 1.61, 95% CI 1.33–1.96). It would be interesting to assess the effect of diuretics vs a normal calcium-low protein-low salt diet, but so far, no studies of this comparison have been performed.
In the review authored by Phillips et al.,28 (patients with calcium-containing kidney stones), citrate therapy significantly reduced the stone size (4 studies, 160 patients: RR 2.35, 95% CI 1.36–4.05) and new stone formation rate (7 studies, 324 patients: RR 0.26, 95% CI 0.10–0.68) compared to the control condition. We could not draw conclusions about the beneficial effect of citrate in patients with hypercalciuria since we were not able to obtain separate data on patients with this underlying metabolic condition.
The risks associated with pharmacological treatment for kidney stones have been reviewed by York et al.,54 and all possible adverse events were described. Only hypokalaemia, hypotension and increases in plasma uric acid were reported in the included studies in the current meta-analysis. The incidence of adverse events reported by Moe55 was much higher (up to 30%) than that reported in the included studies in our systematic review (from 2% to 21%).
ConclusionIn adult patients with IH and recurrent stones, the addition of diuretics to a normal or modified diet probably increases the number of stone-free patients and may reduce the rate of stone formation. It is uncertain whether diuretics increase the occurrence of severe adverse events. There were no studies investigating the effect of pharmacological treatment on urinary symptoms in idiopathic hypercalciuria. We found no studies performed in children. We suggest that better designed, adequately powered clinical trials are required in patients with idiopathic hypercalciuria to determine with greater certainty the effects of pharmacological interventions on these patients compared to diet interventions.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We thank the Cochrane Renal Group and Cochrane Iberoamericana for their help in performing the search strategies. We thank Camila Escobar Liquitay (Cochrane Argentina Instituto Universitario Hospital Italiano) for assistance with updating the searches.