Phytate, or myo-inositol 1,2,3,4,5,6-hexakis dihydrogen phosphate (InsP6), is a naturally occurring phosphorus compound that is present in many foods, mainly legumes, whole grains and nuts. Patients with chronic kidney disease (CKD) have cardiovascular disease mortality up to 30 times higher than the general population. Vascular calcifications (VCs) directly contribute to overall morbidity and mortality, especially in CKD. In part, this high mortality is due to elevated levels of phosphorus in the blood. Therefore, control of dietary phosphorus is essential. Dietary phosphorus can be classified according to its structure in organic phosphorus (plant and animal) and inorganic (preservatives and additives). Plant-phosphorus (legumes and nuts), mainly associated with InsP6, is less absorbable by the human gastrointestinal tract as the bioavailability of phosphorous from plant-derived foods is very low. Recent data indicate that restriction of foods containing plant phosphates may compromise the adequate supply of nutrients that have a beneficial effect in preventing cardiovascular events, such as InsP6 or fibre found in legumes and nuts. Experimental studies in animals and observational studies in humans suggest that InsP6 can prevent lithiasis and VCs and protect from osteoporosis. In conclusion, we need prospective studies to elucidate the potential benefits and risks of phytate (InsP6) through the diet and as an intravenous drug in patients on haemodialysis.
El fitato o myo-inositol-1,2,3,4,5,6-hexakis dihidrogenofostato (InsP6) es un compuesto fosforado de origen natural que está presente en numerosos alimentos, principalmente en legumbres, cereales integrales y frutos secos. Los pacientes con enfermedad renal crónica (ERC) experimentan una mortalidad por enfermedad cardiovascular hasta 30veces mayor que la población en general. Las calcificaciones vasculares (CV) contribuyen directamente en la morbimortalidad general, y de forma especial en la ERC. Esta elevada mortalidad se debe, en parte, a la elevación en los niveles de fósforo en sangre. Por ello, el control de fósforo en la dieta es fundamental. El fósforo dietético puede clasificarse en función de su estructura en fósforo orgánico (origen vegetal y animal) e inorgánico (conservantes y aditivos). El fósforo de origen vegetal (legumbres y frutos secos), principalmente asociado a InsP6, es menos absorbible por el tracto gastrointestinal humano siendo la biodisponibilidad del fósforo procedente de estos alimentos muy baja. Datos recientes indican que la restricción impuesta de alimentos que contienen fosfatos vegetales puede comprometer el aporte adecuado de nutrientes que tienen un efecto beneficioso en la prevención de episodios cardiovasculares, como pueda ser la fibra o al propio InsP6 presente en frutos secos y legumbres. Estudios experimentales en animales y observacionales en humanos sugieren que el InsP6 puede prevenir la litiasis, las CV y proteger de la osteoporosis. En conclusión, creemos necesario realizar estudios prospectivos para elucidar los posibles beneficios y riesgos de una dieta rica en fitato (InP6) en la ERC o de su uso como fármaco intravenoso en pacientes en hemodiálisis.
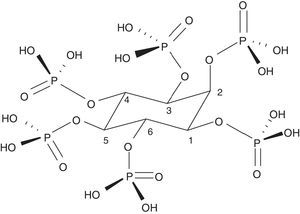
Phytate or myo-inositol-1,2,3,4,5,6-hexakis dihydrogen phosphate (InsP6) is the basis of phytic acid (Fig. 1). It is a natural component widely distributed in the plant kingdom. It serves as a store of phosphate and minerals and contains 75% of the total phosphate of seeds. The main source of InsP6 is in whole grains, legumes, seeds and nuts. These elements are very important for human consumption and constitute 40–60% of the calories ingested in developed and developing countries, respectively. In cereals, it is mainly located in the aleuronic layers and in legumes in the protein bodies of the endosperm or the cotyledon. During germination, InsP6 is hydrolysed allowing the phosphate, magnesium and calcium to be available for the development of the plant. It is, therefore, the main source of plant phosphate. InsP6 is predominantly present in unprocessed foods, as it can be degraded during processing and varying amounts of phosphate inositols may appear with less phosphates (myo-inositol pentaphosphate…).1 Some of them, such as inositol triphosphate (DL-Ins1,4,5 P3), are well-known intracellular messengers, which indicates the great importance that these compounds may have in human biology. The amount of InsP6 that is consumed is very variable and depends on the type of diet. In the Western diet it may range from 0.3 to 2.6g per day, and globally, from 0.180 to 4.569g per day.2 In developing countries and in exclusively vegetarian diets consumption can be very significant; on the other hand, in diets with predominance of “junk food” or with excess meat, typical of the Western diet, it is very poor.1 The Mediterranean diet probably contains an intermediate amount of InsP6 in the diet (1g per day).3 During domestic handling of foods (cooked at about 100°C) InsP6 is quite stable. However, industrial manipulation, in which more extreme conditions are used or phytases are incorporated, its degradation can become very significant.1
Diet phosphateIn patients with chronic kidney disease (CKD), hyperphosphataemia may favour bone-mineral disease (renal osteodystrophy), promote vascular calcification (VC), cardiovascular events, and death.4 Therefore, the control of phosphorus in the diet is fundamental. Dietary protein contains has a considerable amount of phosphorus; however, the phosphate/protein ratio is variable.5 Dietary phosphorus can be classified according to its structure as organic (plant and animal origin) and inorganic (preservatives and additives) phosphorus. In general, 40–60% of phosphorus of animal origin is absorbed,5 whereas plant phosphorus (legumes and nuts), mainly associated with InsP6, is less absorbable by the human gastrointestinal tract.5 When food reaches the digestive tract, hydrolysis mechanisms that release phosphate must occur to make the absorption of phosphates contained in InsP6 possible. Humans lack endogenous phytases, so the presence of phytases in our digestive tract will depend on the intake of plant foods containing active phytases. The presence of these phytases depends on the origin of the food (natural or processed), since during the preparation and cooking of food they are inactivated, or on whether they are introduced into the food during industrial processing (e.g., in the manufacture of bread) to enhance the hydrolysis of InsP6. This hydrolysis may range from 37% to 66%, depending mainly on the presence of phytases.1 Therefore, in Western diets the low presence of phytases means that InsP6 is hardly degraded in the stomach or small intestine, phosphate is not released and therefore the absorption of plant phosphate is low. In contrast, up to 100% of inorganic phosphorus is absorbed from processed foods (such as cheese and some soft drinks like colas).6–8 Therefore, dietary counselling of patients with CKD should not only take into account the absolute content of phosphorus in the diet, but also the chemical structure (inorganic vs. organic phosphate), type (animal vs. plant) and the protein to phosphorus ratio.5,9 One study compared phosphataemia at baseline and after 3 months of receiving dietary advice to avoid foods with inorganic phosphorus additives versus those that continued to receive usual care. At 3 months, the decrease in serum phosphorus levels was greater in the intervention group than in the control group.10 Another recent study compared 9 patients with CKD who received a diet with protein of vegetable origin vs a diet with protein of animal origin; after one week, the vegetarian diet produced a greater reduction of serum phosphate levels ant this was associated to a reduction of circulating FGF23.11 Given this data, it does not seem reasonable to restrict the consumption of foods containing plant phosphate (InsP6), such as nuts, legumes and whole grains,12–14 in patients with CKD. This type of food, in turn, is rich in fibre. In fact, a high fibre diet may have beneficial effects in CKD patients that may have been deprived of for a long time, as demonstrated in several cohort studies15–21 or in the PREDIMED study,22 which suggest that moderate consumption of nuts and high-fibre foods in patients with CKD or high vascular risk could have a significant protective effect in the prevention of cardiovascular events.23
Potential deleterious effects of InsP6Due to its chemical characteristics, InsP6 tends to react with polyvalent cations such as calcium, magnesium, zinc and iron, among others, and this could interfere in the absorption of these minerals. In fact, InsP6 had been classically considered an anti-nutrient for that reason.24,25 However, the beneficial or deleterious effect of InsP6 will vary according to the situation. The inhibitory effect of InsP6 on metal absorption is neutralized by the presence of other nutrients such as organic acids, ascorbic acid, food fermentation products, etc., competing with phytic acid for the binding of minerals and trace elements. Therefore, in the context of a balanced diet in developed countries, there is no evidence that InsP6 has any detrimental effect in well-nourished populations.26–30 The situation in developing countries is different, with mainly vegetarian diets, very poor in meat, dairy products and other nutrients; in this condition it is possible that a high amount of InsP6 in the diet might contribute to the malabsorption of calcium, magnesium, iron and zinc. For this reason, the development of foods with lower InsP6 content is encouraged in these countries, mainly by the addition of phytases of bacterial origin.31,32
Beneficial effects of InsP6The source of phosphate in diet is important, phosphate from vegetables will have less impact in the body phosphate balance than other sources of phosphate. In addition, the presence of InsP6 per se, may have beneficial effects through its ability to inhibit pathological calcifications (lithiasis, VC…), also because of its antioxidant effect and its potential effect on the prevention of certain cancers. With a pH around 6–7, the InsP6 is strongly negatively charged, and since there in no trans-cellular transporters of InsP6, its intestinal absorption would be restricted to a passive mechanism via the para-cellular pathway. Relatively recent studies in humans and rats have found that intact InsP6 is absorbed in a small proportion (<2%), and the presence in blood and urine is almost totally dependent on intestinal absorption.1,33,34 In traditional Mediterranean diet the daily intake of InsP6 is approximately 1g.3 However, phytate absorption is saturable and there are maximum plasma levels that cannot be exceeded despite increased oral intake. Nevertheless, InsP6 concentrations achieved with oral intake may produce a natural basal protection against pathologies related with calcification. The InsP6 levels will be reduced if this type of diet is changed by dietary patterns in which the presence of fibre is very scarce. In about 15–20 days of a diet without InsP6, the levels are reduced to virtually undetectable. InsP6 levels in urine have been shown to be representative of InsP6 consumption in the diet.35 In one report, the author doubts about the natural presence of InsP6 in urine and plasma, although the differences of opinion seem to be derived from results obtained using different analytical methods used in its measurement, which are complex and have long hampered its study in biological matrices.36–39
Antioxidant effectOne important feature of InsP6 is the observed antioxidant effect.40,41 This is mainly based on its ability to form highly stable complexes with iron, which prevents its interaction with hydrogen peroxide and the formation of hydroxyl radicals. This mechanism is different from that of other antioxidants such as ascorbic acid or beta-carotene, which act as scavengers. Although the antioxidant effect in in vitro conditions is clear, in vivo evidence is scarce, so further studies are needed to elucidate the impact of the antioxidant effect of InsP6.1
Anticancer activityThe beneficial effect of InsP6 on several types of cancer (mainly colon, but also liver, lung, breast, prostate, skin and soft tissue) has been demonstrated in studies on cell lines and in some animal models. However an in vivo therapeutic effect in humans has not been demonstrated.42
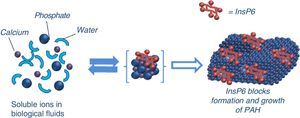
Role of InsP6 as an inhibitor of vascular calcificationsPathological crystallisation is a process that takes place when undesirable crystalline solids are produced under physiological conditions of organisms. If these solids involve calcium salts they are called calcifications. These include renal lithiasis, dental calculus, chondrocalcinosis, calcinosis cutis and, finally, the VCs.1 A calcium phosphate mineral called hydroxyapatite (HAP) is present in VCs as well as in bone. VCs directly contribute to overall morbidity and mortality, and especially in patients with CKD.4,43,44 Dialysis patients have calcium scores 2–5 times higher than subjects of the same age with normal kidney function.45 The presence of calcifications in the arterial wall is associated with 3–4 times increased risk of coronary heart disease, stroke and heart failure.46 Calciphylaxis is a rare but devastating disease that can affect 4% of haemodialysis patients. It begins with the calcification of the small peripheral vessels and quickly spreads. It is the most severe form of VC in patients on dialysis and affects only the middle layer of the vessel. Its natural course leads to the development of very painful necrotic ulcers as a result of the VC process. It has an annual mortality of 45–80%.47 There are still no treatments approved specifically for this indication.48
The first studies published on crystallisation inhibitors date back to the 1960s, by Fleisch and Bliznakov.49 The first to be discovered was inorganic pyrophosphate, which is a natural polyphosphate product of the degradation of many physiological reactions (derived from AMP), present in blood and urine. Alkaline phosphatase reduces both its plasma and tissue levels (so an increase in alkaline phosphatase may contribute to increased VC). It is hydrolysed when given orally so, bisphosphonates were developed, which cannot be hydrolysed. They are resistant to the effect of alkaline phosphatase50 and therefore can penetrate the bone. Bisphosphonates consist of 2 phosphonate groups attached to the same carbon atom and 2 R side-chains, one of which is normally an alcohol group. The potential role of bisphosphonates in the prevention of VC has been described.51,52 In addition to their effect on crystallisation, they may also have an inhibitory effect on bone resorption by osteoclasts; therefore, they are also useful in the treatment of osteoporosis. One of the disadvantages is its long half-life on the surface of the bone, which can cause a dynamic bone disease in CKD patients.50 Similarly InsP6 also seems to act as a crystallisation inhibitor (Fig. 2), but according to results from experimental studies, InsP6 has a higher potency than pyrophosphate and bisphosphonates, as we will described later. The mechanism of action may be either at the nucleation level (adsorption at the surface of the nucleus) or during the growth or aggregation of the crystals, thus retarding or preventing the crystallisation of the supersaturated substance. However, the adsorption to the crystal surface may prevent its dissolution (therefore, the same substance could hinder VCs and, at the same time decrease bone resorption, thus protecting from osteoporosis).
Experimental studies in animals demonstrating the ability of InsP6 to inhibit vascular calcificationsEarly experimental studies in rats showed that dietary InsP6 significantly reduces aortic calcifications associated with ageing. Ten week old Male Wistar rats were treated and randomly assigned to 4 diet groups, 2 of them rich in InsP6 and 2 without InsP6. At 76 weeks, all the rats were sacrificed and aortas, hearts, kidneys, livers and femurs were obtained for chemical analysis. The most important differences were found in the calcium content of the aorta. The groups fed a diet rich in InsP6 had calcium levels approximately 40% lower than those on diets without InsP6.53
The ability of InsP6 to inhibit VCs in rats subjected to calcinosis has been demonstrated by several methods. By inducing hypertension (with nicotine) and hypercalcaemia (with vitamin D at high doses), calcifications were induced in renal tissue of Wistar rats that had been fed a diet without InsP6. The animals developed significant deposits of calcium in renal papillae, renal interstitium, renal tubules and vessels. By contrast, rats that received InsP6 transdermally did not develop calcifications.54 Another study using the same animal model of calcinosis showed a reduction of calcification in aorta and heart tissue, applying InsP6 topically.55 Finally, transdermal InsP6 demonstrated its effectiveness in another experimental calcinosis cutis model caused by the subcutaneous injection of KMnO4.56 Using this calcinosis cutis model, rats received containing InsP6 sodium at 1% or enriched with carob germ (rich in InsP6) versus a group without InsP6, and another group without InsP6 but treated with subcutaneous etidronate. The results showed that that only those with adequate levels of InsP6 had a reduction of dystrophic calcifications.57 In another experimental model, calcinosis was induced in male Sprague–Dawley rats (n=6 per group) using very high doses of vitamin D (500,000IU/kg) given at 0, 24 and 48h. One group received placebo, another etidronate (0.825μmol×kg−1×day−1) and the third group received InsP6 (0.825μmol×kg−1×day−1). At 96h, rats were sacrificed and the calcium content of aortas and hearts was measured. It was found that Insp6-treated rats, but not those treated with etidronate, had less aortic calcifications than placebo-treated rats.58
In more recent studies, 40 male Sprague–Dawley rats were divided into 3 groups that were respectively treated with 1mg/kg of SNF472 (an intravenous formulation of InsP6), 15mg/kg of oral cinacalcet and 400mg/kg of sodium thiosulfate. Calcification was induced by the administration of 75,000IU/kg of vitamin D3 3 days after starting treatments. The rats were sacrificed at day 14 and the aortas and hearts were used to analyse the calcium content. Intravenous administration of SNF472 reduced calcifications by 60% in the aorta and by 68% in myocardial tissue. Cinacalcet caused a statistically significant reduction of VC by 24%, but not thiosulfate, so the potency of intravenous InsP6 is higher than that of sodium thiosulfate or cinacalcet.59 An in vitro study showed the high affinity of SNF472 on hydroxyapatite crystals.60
Observational studies linking InsP6 consumption to decreased vascular calcifications in humansA cross-sectional study in elderly subjects evaluated the relationship between levels of urinary InsP6 (which represents InsP6 consumption) and valvular calcifications as assessed by echocardiography. The population was divided into tertiles according to the urinary levels of InsP6. Those with higher levels of InsP6 had the mitral valve less calcified and also had less frequency of diabetes and hypercholesterolaemia. In the multivariate analysis, age, blood phosphate, total leukocytes and urinary InsP6 were independent predictors of mitral valve calcification. In addition, there was an inverse correlation between InsP6 levels and these calcifications.61
In a prospective cross-sectional study that we have conducted recently, the abdominal aorta calcifications (AAC) were assessed by single lateral abdominal plaque in 69 patients with CKD stages 2 and 3 from our outpatients clinics. A dietary survey was conducted to assess InsP6 intake and the urine InsP6 levels were determined. The study population was divided into 2 groups based on whether their AAC score was above or below the median (AAC of 6). Patients without calcifications were younger, had lower pulse pressure, less frequency of cardiovascular disease, increased intake of InsP6 and greater elimination of InsP6 in urine. Among the foods rich in InsP6 evaluated, it was found that lentil consumption was higher among patients with less calcifications. In the multivariate analysis, age, previous cardiovascular disease and urinary InsP6 (or lentil consumption) were independently associated with AAC. It may be that the beneficial result of the lentils intake was due to the fact that, among InsP6 rich foods, lentils was the most frequently consumed.62
Role of InsP6 in other pathological calcificationsInsP6 has demonstrated its ability to inhibit the crystallisation of calcium oxalate and calcium phosphate in urine. The intake of InsP6 and the physiological levels have been correlated with a lower incidence and/or prevalence of renal lithiasis. Although we will not review this in depth, the literature is abundant.54,63–76 Several experimental studies in rats have demonstrated the potent protective effect of InsP6 in both calcifications of intrapapillary tissue and in urine itself.57 In patients with calcium oxalate lithiasis with active lithogenic factors, an improvement was observed after 15 days of a diet rich in InsP6.76 A study evaluating the association between dietary factors and risk of symptomatic renal lithiasis in 96,245 women during 8 years demonstrated that a high intake of InsP6 reduced the incidence of renal lithiasis.77 Salivary concentration of InsP6 has been inversely correlated with the incidence of sialolithiasis,78 and its efficacy in preventing plaque formation has been demonstrated in clinical trials.79
Role of InsP6 in osteoporosisInsP6 may play a role in protecting against osteoporosis. A densitometry study of 157 postmenopausal women showed that the 70 patients who had low levels of InsP6 in urine (related to low InsP6 consumption) had a greater bone mass loss at the lumbar spine after 12 months than those with high InsP6 levels.80 In another study, women that consumed foods rich in InsP6 more than 2 times per week had greater bone density in calcaneus, lumbar spine and femur than those who did so once a week or never.81 A possible protective effect of InsP6 from osteoporosis may be through a physical–chemical mechanism (adsorption to the side surface of PAH crystals), hindering the dissolution of PAH. In addition InsP6 could also have an effect on bone cells. In a study with MC3T3-E1 osteoblast cultures, InsP6 was found to inhibit mineralisation of the growing crystal by binding to the their negatively charged phosphates and by inhibiting osteopontin expression; however, the expression of other osteoblastic differentiation markers such as alkaline phosphatase, sialoprotein, and osteocalcin were not affected. These data suggest that InsP6 may participate in the regulation of bone mineralisation by acting directly at extracellular level and serving as a specific cell signal that modulate osteopontin gene expression.82 In an in vitro study on human cell lines (peripheral blood circulating mononuclear cells and RAW 264.7 osteoclast-like cells), InsP6 was found to selectively inhibit osteoclastogenesis.83 Possibly InsP6 and bisphosphonates interacts with bone through different mechanisms.50 It is possible that InsP6 is metabolised by phosphatases avoiding a prolonged permanence in bone, as opposed, bisphosphonates; this would provide a right balance between the instability of pyrophosphate and the long biological half-life of bisphosphonates.
Use of InsP6 as a treatment in humansUse of InsP6 as a nutritional supplementThere are products that have been marketed for years as nutritional, vitamin or nutraceutical supplements, which contain InsP6 in the form of phytin, which is its calcium salt. This product is considered safe, classified as generally recognised as safe (GRAS) by the FDA, and it is included in Chemical Abstract.
In Spain there are InsP6 enriched biscuits. There are several products presented as capsules containing InsP6 together with vitamin A and zinc used for the prevention of calcium stones. A product containing InsP6 together with methionine has recently been marketed to acidify urine and protect against calcium phosphate lithiasis, which develops at high urine pH. A mouthwash for plaque prevention has also been produced.
Use of intravenous InsP6 as a drug for patients on haemodialysisThere is currently no medication approved for the treatment of VCs. SNF472 is an intravenous formulation of InsP6 that is being developed for 2 indications: reduction of cardiovascular events in patients on dialysis and for the treatment of calciphylaxis. While InsP6 intake may lead to physiological levels that provide basal natural protection, the treatment of pathological VCs may require exposure to high InsP6 levels. SNF472 is being developed for this purpose. Two phase 1 clinical trials have been conducted in which its safety and tolerability have been demonstrated in healthy volunteers and in haemodialysis patients at supraphysiological concentrations.84,85 A high dose of InsP6 could produce hypocalcaemia: however, In vitro haemodialysis studies demonstrate that this effect can be neutralised by pre-filter administration of SNF472; and, given its poor clearance therapeutic levels are achieved.86,87
ConclusionIn CKD patients, the consumption of food containing plant phosphates (legumes, nuts, fibre…) produces a modest increase in serum phosphate that is comparatively less than that produced by ingestion of phosphates of animal or inorganic origin. In addition, food containing plant phosphates also has beneficial effects by providing fibre and InsP6. Experimental studies in animals and observational studies in humans suggest that InsP6 can prevent lithiasis, VCs and protect against osteoporosis. In addition, it may have antioxidant and anticancer effects. InsP6 is used with nutritional supplements for prevention of lithiasis. The initial results with the investigational drug SNF472 are very promising and support the continuation of the research in this line to obtain the first drug with indication for the prevention of VC in patients on haemodialysis and as treatment of calciphylaxis.
Key concepts- •
In patients with chronic kidney disease (CKD), consumption of plant foods containing phosphates (legumes, nuts…) increases blood phosphorus levels less than phosphates of animal origin or foods with inorganic phosphate additives. This is because plant foods containing phosphates are mainly in the form of phytate (InsP6), less absorbable by the human gastrointestinal tract because we lack endogenous phytases.
- •
Foods containing plant phosphates can also provide beneficial elements for health, such as fibre and InsP6.
- •
Experimental studies in animals and observational studies in humans suggest that InsP6 can prevent lithiasis, vascular calcifications (VCs) and protect against osteoporosis.
- •
New prospective studies will be needed to elucidate the potential benefits and risks of an InsP6-rich diet in patients with CKD.
- •
The initial results with the investigational drug SNF472 are very promising and favour further research to obtain the first drug with indication in the prevention of VC in patients on haemodialysis or in the treatment of calciphylaxis.
JP is co-founder and CEO of Sanifit Laboratories, a company that is developing SNF472. JP and FG are coinventors of the WO2010018278 patent. The rest of the authors declare no conflict of interest.
Please cite this article as: Buades Fuster JM, Sanchís Cortés P, Perelló Bestard J, Grases Freixedas F. Fosfatos de origen vegetal, fitato y calcificaciones patológicas en la enfermedad renal crónica. Nefrologia. 2017;37:20–28.