Posterior reversible encephalopathy syndrome is a clinical and radiological entity with acute or subacute neurological presentation associated with brain lesions that primarily affect the white matter of the posterior regions. It is often associated with the rapid onset of severe hypertension and/or with kidney failure (acute and chronic), but it has also been reported as a neurological complication in several medical conditions. In recent years, there has been an increase in the number of cases and related publications due to the advance of diagnostic imaging techniques. The characteristic radiological finding includes hyperintense lesions in T2- and FLAIR-weighted magnetic resonance imaging, which are often bilateral and located in the posterior cerebral regions and correspond to areas of vasogenic oedema.
Little is known about the pathophysiology of posterior reversible encephalopathy syndrome. The most accepted theory, especially in cases with associated hypertension, is the loss of cerebral self-regulation which leads to the onset of vasogenic oedema. The main feature of this syndrome is the reversibility of both symptoms and cerebral lesions with an early and appropriate diagnosis.
Despite the frequent association with kidney failure and severe hypertension, there are few cases reported in patients on peritoneal dialysis. This article presents a review of PRES in peritoneal dialysis patients in the published literature.
El síndrome de encefalopatía posterior reversible es una entidad clínico-radiológica con presentación neurológica aguda o subaguda, asociada a la presencia de lesiones que afectan sobre todo a la sustancia blanca de las regiones cerebrales posteriores. Se asocia principalmente con hipertensión severa de rápido desarrollo, o con insuficiencia renal (aguda o crónica), aunque se ha descrito también como una complicación neurológica de varias entidades médicas. En los últimos años se está produciendo un aumento en el número de casos y publicaciones relacionadas, debido al avance de las técnicas diagnósticas de imagen. El hallazgo radiológico característico es la presencia en la resonancia magnética de lesiones hiperintensas en las secuencias T2 y FLAIR, frecuentemente bilaterales y localizadas en las regiones cerebrales posteriores, que se corresponden con zonas de oedema vasogénico.
Poco se conoce de la fisiopatología del síndrome de encefalopatía posterior reversible. La teoría más aceptada, sobre todo en los casos con hipertensión asociada, es la de la pérdida de la autorregulación cerebral, que conduce a la aparición de oedema vasogénico. Su característica principal es la reversibilidad, tanto de la clínica como de las lesiones cerebrales, con un diagnóstico precoz y adecuado.
Pese a la frecuente asociación con insuficiencia renal y con hipertensión severa, son pocos los casos publicados en pacientes de diálisis peritoneal. Presentamos aquí una revisión del síndrome de encefalopatía posterior reversible en pacientes en diálisis peritoneal y de la casuística publicada.
Posterior reversible encephalopathy syndrome (PRES) is a clinical-radiological condition with acute or subacute neurological presentation, associated with the presence of brain lesions predominantly affecting white matter in the posterior regions. The main characteristic is the reversibility of both clinical symptoms and the brain lesions, if a correct diagnosis is made early.1–6
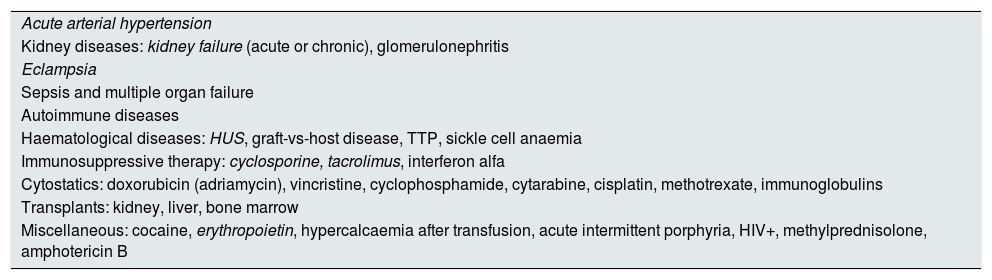
PRES is mainly associated with severe, rapid-onset arterial hypertension (HTN) or with kidney failure (acute or chronic), although it has also been reported as a neurological complication of several medical conditions, such as eclampsia, vasculitis and connective tissue diseases, blood diseases, liver disease, hypercalcaemia, the use of erythropoietin and a wide range of immunosuppressive or cytotoxic drugs1–6 (Table 1).
Triggering factors associated with PRES.
| Acute arterial hypertension |
| Kidney diseases: kidney failure (acute or chronic), glomerulonephritis |
| Eclampsia |
| Sepsis and multiple organ failure |
| Autoimmune diseases |
| Haematological diseases: HUS, graft-vs-host disease, TTP, sickle cell anaemia |
| Immunosuppressive therapy: cyclosporine, tacrolimus, interferon alfa |
| Cytostatics: doxorubicin (adriamycin), vincristine, cyclophosphamide, cytarabine, cisplatin, methotrexate, immunoglobulins |
| Transplants: kidney, liver, bone marrow |
| Miscellaneous: cocaine, erythropoietin, hypercalcaemia after transfusion, acute intermittent porphyria, HIV+, methylprednisolone, amphotericin B |
HUS, haemolytic uraemic syndrome; TTP, thrombotic thrombocytopenic purpura.
The most common are in italics.
Despite the frequent association with severe HTN and with kidney failure, it is surprising how few cases have been published in the context of peritoneal dialysis (PD); still, some authors have pointed out that this dialysis technique could be another triggering factor of the symptoms.7–9
This was reported for the first time in 199610 and its nomenclature became the subject of much debate.11 Initially named posterior reversible leukoencephalopathy syndrome, the symptoms were considered a variant of hypertensive encephalopathy. However, as brain involvement is not always limited to white matter, the use of the term posterior reversible encephalopathy syndrome (PRES) is becoming more widely accepted. In contradiction to the name, the disease may also affect anterior areas of the brain and may not be reversible.1,12,13
Its incidence and prevalence are not entirely known, although in the last few years there has been an increase in the number of related publications and cases, particularly due to the advance in diagnostic imaging techniques. It seems to be slightly less prevalent in women,9,12 and very common in children with kidney failure.14,15 While it was thought that the symptoms were monophasic, it can recur if the precipitating context is repeated.16,17
Clinical symptomsThe most common symptomatology includes intense headaches, somnolence or restlessness, mental confusion, visual impairment (generally blurred vision, but also anopsia or cortical blindness), focal neurological symptoms and seizures (reported in 90% of cases; on many occasions these are the first symptom), either focal with secondary generalisation or quite generalised from the beginning. Other less common symptoms include nausea, vomiting and non-convulsive status epilepticus (which should be suspected in the event of repeated stereotyped movements). The level of consciousness progressively deteriorates and may give rise to stupor or coma. Clinical symptoms develop in the space of a few hours and can persist for weeks depending on their severity and how fast treatment is started.1–6,9
Hypertension is present in the majority of cases, whether normotensive patients with an acute increase in blood pressure or with chronic exacerbated HTN. It has been suggested that rapidity of instauration rather than the absolute value of the BP readings is what triggers the disease. The systolic blood pressure range from 170 to 200mmHg (although 10–30% of cases may not reach these levels).1,4,6,8 A significant proportion of published cases report have acute or chronic kidney failure as an underlying disease or in association with other intercurrent processes.4
The typical radiological finding is the presence on the MRI of hyperintense lesions in the sequences T2 and FLAIR, generally (although not always) bilateral and located in the posterior brain regions, which correspond to areas of vasogenic oedema. These lesions are reversible in a large proportion of cases, after the aetiological factors involved are corrected.
Four different patterns of brain involvement have been described: the typical and most common posterior pattern with occipitoparietal lesions; a holohemispheric pattern with a linear distribution that spans practically all the lobes, except for the temporal lobes; an upper frontal pattern with variable temporal involvement; and a partial asymmetrical pattern with no posterior involvement and with asymmetrical involvement.9,12
PathophysiologyNot much is known about the pathophysiology of PRES.1–6,8,9 The most widely accepted theory, particularly in cases with associated HTN, is the loss of brain self-regulation which, under normal conditions, keeps the cerebral blood flow constant by vasoconstriction of the brain arterioles, thus protecting the brain from acute changes in blood pressure. With increased blood pressures, this self-regulation fails, eliciting arteriolar vasodilation and endothelial dysfunction which cause capillary transudation and disruption of the blood–brain barrier, giving rise to accumulation of fluid in the surrounding brain tissue. The greater involvement of the posterior brain areas is due to their smaller perivascular sympathetic innervation (responsible for the protective myogenic response of the brain arterioles), making them more susceptible to changes in blood pressure.
There are patients who develop this syndrome with only slight increases in blood pressure. In these cases, other circumstances usually concur which elicit or predispose the blood–brain barrier to damage or interfere with the sympathetic nervous system, such as sepsis, electrolyte disturbances, high temperature or the use of certain drugs.
When PRES is associated with drugs (mainly immunosuppressants and cytotoxins) with no associated HTN, the pathogenesis is less certain. A possible direct cytotoxic effect on the endothelium is hypothesised for these cases, or neurotoxicity promoted by other intercurrent factors such as biochemical changes, or systemic oedema.2,3
Another alternative theory is that PRES is the consequence of an inflammatory status which causes endothelial dysfunction. This would explain the association with processes such as sepsis, autoimmune diseases or transplants. In these cases, the vasoconstriction caused by the inflammatory status would work together with the initial vasoconstriction of the self-regulation mechanism and the resulting ischaemia would be the cause of the endothelial dysfunction.
Recently there has been some debate about the possible role of hypomagnesaemia as a new triggering factor for PRES. Because of its known neuroprotective function, magnesium sulphate is usually and effectively used to prevent seizures in pre-eclampsia. Recent articles have shown the common association between hypomagnesaemia and PRES, and the PRES-enabling mechanism of calcineurin inhibitors has been attributed to this electrolyte imbalance.18,19
Kidney failure, as well as HTN, is another factor often related to PRES, particularly in the paediatric population.4,16,17,20 The pathogenesis in this situation seems multifactorial: uraemic toxins and poor water and sodium control, and the frequent association with hypoalbuminaemia and hypertension lead to lower osmotic pressure and to raised hydrostatic pressure, which increase vascular permeability. In this context, susceptibility to any endothelial involvement increases, whether due to increased blood pressure or cytotoxicity. The usual associated treatments also have an impact, such as erythropoiesis-stimulating agents (ESA), which contribute to hypertension, or calcineurin inhibitors.
Diagnosis and differential diagnosisNeurological symptoms may indicate other conditions like cavernous sinus thrombosis, demyelinating diseases, intracranial haemorrhage, encephalitis or stroke. The sudden presentation makes us suspect ischaemic processes which must be ruled out, and may not be distinguished in CAT scans or conventional MRI. In PRES, the calcarine regions and the paramedian occipital lobe structures are not usually affected and the lesions may be seen simultaneously in different cerebral zones that are dependent on various brain arteries.3 The accuracy of the diagnosis is important, since the control of HTN in the acute phase of stroke is not the same as in PRES.
Posterior reversible encephalopathy syndrome and peritoneal dialysisDespite the numerous cases published on patients with kidney failure, many of them on haemodialysis,13 there are few reported cases of patients on PD. This is surprising given that, as well as kidney failure and the typical HTN, patients are usually overhydrated and in this dialysis technique albumin is lost through the dialysate. One possible explanation which comes to mind could be the relative haemodynamic stability of patients on PD compared to the intermittent changes patients on haemodialysis undergo.
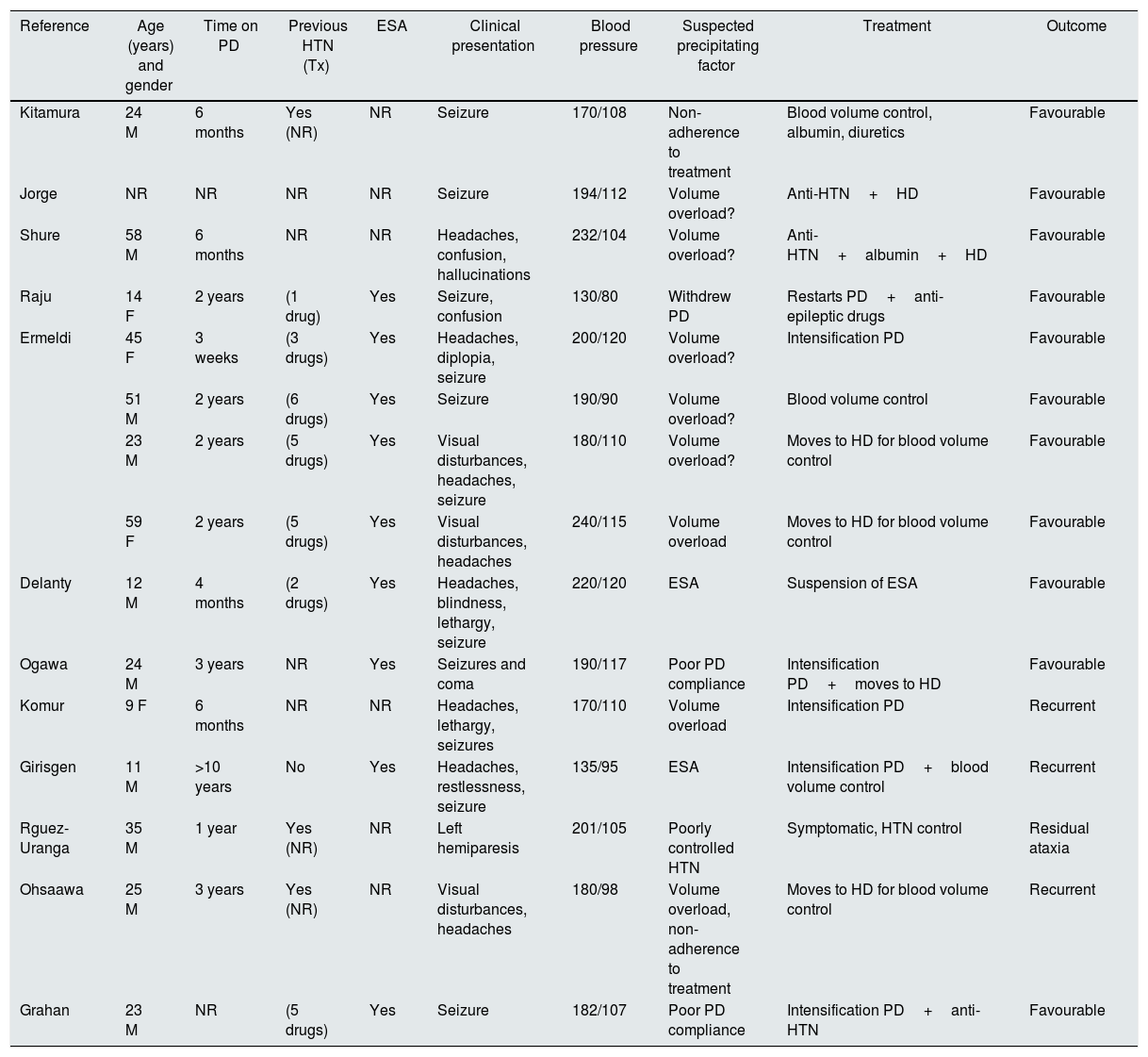
On reviewing the literature, we have found a little more than a dozen cases of PRES in PD patients7–9,16,17,20–26 (Table 2). The majority were males (in the general literature are predominantly females affected) and young people (aged between 20 and 50 years), with only five cases in children and three in people over 50. The length of time on PD prior to the PRES episode is almost one year (range between three weeks and three years). Practically all cases presented with seizures as an initial symptom and the majority received anti-HTN treatment. In several cases, volume overload and poor dialysis compliance stand out as enabling or aggravating factors, and in many of them erythropoietin is involved. There were three cases of recurring PRES.16,17,20 The increased dose of PD and ultrafiltration (or even the shift to haemodialysis) is reported as part of the treatment in several patients. In general, all patients recovered without sequelae.
Summary of PRES and PD cases in the literature.
| Reference | Age (years) and gender | Time on PD | Previous HTN (Tx) | ESA | Clinical presentation | Blood pressure | Suspected precipitating factor | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Kitamura | 24 M | 6 months | Yes (NR) | NR | Seizure | 170/108 | Non-adherence to treatment | Blood volume control, albumin, diuretics | Favourable |
| Jorge | NR | NR | NR | NR | Seizure | 194/112 | Volume overload? | Anti-HTN+HD | Favourable |
| Shure | 58 M | 6 months | NR | NR | Headaches, confusion, hallucinations | 232/104 | Volume overload? | Anti-HTN+albumin+HD | Favourable |
| Raju | 14 F | 2 years | (1 drug) | Yes | Seizure, confusion | 130/80 | Withdrew PD | Restarts PD+anti-epileptic drugs | Favourable |
| Ermeldi | 45 F | 3 weeks | (3 drugs) | Yes | Headaches, diplopia, seizure | 200/120 | Volume overload? | Intensification PD | Favourable |
| 51 M | 2 years | (6 drugs) | Yes | Seizure | 190/90 | Volume overload? | Blood volume control | Favourable | |
| 23 M | 2 years | (5 drugs) | Yes | Visual disturbances, headaches, seizure | 180/110 | Volume overload? | Moves to HD for blood volume control | Favourable | |
| 59 F | 2 years | (5 drugs) | Yes | Visual disturbances, headaches | 240/115 | Volume overload | Moves to HD for blood volume control | Favourable | |
| Delanty | 12 M | 4 months | (2 drugs) | Yes | Headaches, blindness, lethargy, seizure | 220/120 | ESA | Suspension of ESA | Favourable |
| Ogawa | 24 M | 3 years | NR | Yes | Seizures and coma | 190/117 | Poor PD compliance | Intensification PD+moves to HD | Favourable |
| Komur | 9 F | 6 months | NR | NR | Headaches, lethargy, seizures | 170/110 | Volume overload | Intensification PD | Recurrent |
| Girisgen | 11 M | >10 years | No | Yes | Headaches, restlessness, seizure | 135/95 | ESA | Intensification PD+blood volume control | Recurrent |
| Rguez-Uranga | 35 M | 1 year | Yes (NR) | NR | Left hemiparesis | 201/105 | Poorly controlled HTN | Symptomatic, HTN control | Residual ataxia |
| Ohsaawa | 25 M | 3 years | Yes (NR) | NR | Visual disturbances, headaches | 180/98 | Volume overload, non-adherence to treatment | Moves to HD for blood volume control | Recurrent |
| Grahan | 23 M | NR | (5 drugs) | Yes | Seizure | 182/107 | Poor PD compliance | Intensification PD+anti-HTN | Favourable |
ESA, erythropoiesis-stimulating agents; F, female; HD, haemodialysis; HTN, arterial hypertension; M, male; NR, not recorded; PD, peritoneal dialysis; Tx, treatment.
PRES treatment includes antihypertensive drugs, with the aim to achieve mean blood pressures between 105 and 125mmHg, anticonvulsant drugs, the suspension of drug that could be related with the disease, the intensification of the dialysis and ultrafiltration dose (even changing the form of dialysis if necessary) or the administration of albumin and magnesium. Resolution without sequelae will depend on the speed at which treatment is established and its intensity.
PRES must be considered in those patients who present with acute neurological clinical symptoms (intense headaches, seizures, visual disturbances and changes in the level of consciousness), especially if it is associated with HTN or volume overload.
PRES can be confirmed quickly using magnetic resonance imaging. Administering appropriate treatment quickly is the key to achieving the reversibility of the symptoms and avoiding permanent brain damage.
Patients on PD possess several factors which in theory make them more susceptible to suffering from these symptoms. However, only few cases have been reported to date in this population, so they cannot be described as a risk group.
The authors declare that they have no conflict of interests.
Please cite this article as: Moreiras-Plaza M, Fernández-Fleming F, Azkárate-Ramírez N, Nájera-de la Garza W, Martín-Baez I, Hernansanz-Pérez M. Diálisis peritoneal: ¿un factor de riesgo o de protección para la encefalopatía posterior reversible (PRES)? Revisión de la literatura. Nefrologia. 2018;38:136–140.