Protein-energy wasting (PEW) is associated with increased mortality and differs depending on the chronic kidney disease (CKD) stage and the dialysis technique. The prevalence in non-dialysis patients is understudied and ranges from 0 to 40.8%.
ObjectiveTo evaluate the nutritional status of a group of Spanish advanced CKD patients by PEW criteria and subjective global assessment (SGA).
Patients and methodsCross-sectional study of 186 patients (101 men) with a mean age of 66.1±16 years. The nutritional assessment consisted of: SGA, PEW criteria, 3-day dietary records, anthropometric parameters and bioelectrical impedance vector analysis.
ResultsThe prevalence of PEW was 30.1%, with significant differences between men and women (22.8 vs. 33.8%, p<0.005), while 27.9% of SGA values were within the range of malnutrition. No differences were found between the 2 methods. Men had higher proteinuria, percentage of muscle mass and nutrient intake. Women had higher levels of total cholesterol, HDL and a higher body fat percentage. The characteristics of patients with PEW were low albumin levels and a low total lymphocyte count, high proteinuria, low fat and muscle mass and a high Na/K ratio.
The multivariate analysis found PEW to be associated with: proteinuria (OR: 1.257; 95% CI: 1.084–1.457, p=0.002), percentage of fat intake (OR: 0.903; 95% CI: 0.893–0.983, p=0.008), total lymphocyte count (OR: 0.999; 95% CI: 0.998–0.999, p=0.001) and cell mass index (OR: 0.995; 95% CI: 0.992–0.998).
ConclusionMalnutrition was identified in Spanish advanced CKD patients measured by different tools. We consider it appropriate to adapt new diagnostic elements to PEW criteria.
El desgaste proteico energético (DPE) se asocia a mayor mortalidad y difiere dependiendo del estadio de la enfermedad renal y de la técnica de diálisis. Su prevalencia en pacientes sin diálisis se encuentra poco estudiada y oscila entre el 0 y el 40,8%.
ObjetivoEvaluar el estado nutricional según criterios de DPE y por valoración global subjetiva (VGS) de un colectivo de pacientes españoles con enfermedad renal crónica avanzada (ERCA).
Pacientes y métodosEstudio transversal de 186 pacientes (101 hombres) con edad media de 66,1±16 años. Se realizó evaluación nutricional mediante: VGS, criterios de DPE, registro dietético de 3 días, parámetros antropométricos y bioimpedancia vectorial.
ResultadosUn 30,1% presentaba DPE, con diferencias significativas entre hombres y mujeres (22,8 vs. 33,8%; p<0,005) y un 27,9% tenía valores de VGS en rangos de desnutrición. Sin diferencia entre los 2 métodos estudiados. Los hombres presentaron mayores niveles de proteinuria, porcentaje de masa muscular e ingesta de nutrientes. Las mujeres tuvieron mayores niveles de colesterol total, HDL y porcentaje de masa grasa. Las características de los pacientes con DPE fueron: bajos valores de albúmina y recuento total de linfocitos, elevada proteinuria, baja masa grasa, baja masa muscular y cociente Na/K elevado.
El análisis multivariante mostró asociación de DPE con proteinuria (OR: 1,257; IC 95%: 1,084-1,457; p=0,002), porcentaje de ingesta lipídica (OR: 0,903; IC 95%: 0,893-0,983; p=0,008), recuento total de linfocitos (OR: 0,999; IC 95%: 0,998-0,999; p=0,001) y el índice de masa celular (OR: 0,995; IC 95%: 0,992-0,998).
ConclusiónExiste malnutrición en población española con ERCA, medida por diferentes herramientas. Consideramos conveniente adecuar nuevos elementos diagnósticos a los criterios de DPE.
Protein wasting (PEW) is defined as a pathological state in which there is a decrease in protein and energy stores.1 Gracia et al. translates this term into Castilian as “desgaste proteico energético (DPE)” emphasizing that this term gives equal importance to malnutrition and increased catabolism.2
The PEW increases the risk of cardiovascular mortality. This has been demonstrated in patients on maintenance hemodialysis and in patients who initiate dialysis techniques.3,4 In patients with chronic renal disease (CKD) not on dialysis, the decrease in serum albumin levels and total lymphocyte counts (TLC) are associated with an increase the risk of mortality.5
The prevalence of malnutrition varies according to the renal disease stage, the dialysis technique and the methodology used for its diagnosis. Thus, in hemodialysis patients using the subjective global assessment (SGA), the prevalence of malnutrition is 28–80%.6,7 In Spain, using the criteria of the International Society of Renal Nutrition and Metabolism (ISRNM) the prevalence is 37.7%8 and in peritoneal dialysis (PD), using SGA, the figures are between 11.3 and 71.5.9,10 In chronic kidney disease not in dialysis, there are very few studies evaluating the presence of malnutrition, and most of them use SGA and the malnutrition-inflammation scale (MIS); none have been performed in the patient population in Spain but their prevalence ranges from 0 to 40.8%.5,11–13
The nutritional status of the renal patient may be assessed using different methods. For this reason, the ISRNM has proposed one method for the diagnostic for PEWs, which include 4 categories (biochemistry, body mass, muscle mass and intake), as well as the possibility to include other additional measurements such as inflammatory markers or bioimpedance parameters.1
Given the fact that there is no study evaluating the presence of PWE in advanced CKD in Spain and knowing that advanced CKD has an impact on subsequent stages of renal replacement therapy (RRT), it was decided to perform the present study under the hypothesis that in advanced CKD patients the long-term prognosis of nutritional state can be modified using adequate diagnosis and subsequent intervention so the progressive nature of the PEW is prevented.
The objective of the present study is to evaluate the nutritional status of a group advanced CKD patients in Spain using the criteria of PEW from both the ISRNM1 and SGA.14
Patients and methodsPatientsThis is cross-sectional study conducted in 186 patients with advanced CKD. The population was selected from patients attending the advanced CKD outpatient clinic of the Nephrology Service, Hospital Universitario La Paz (Madrid, Spain).
Inclusion criteria were as follows: age >18 years, CKD stage 4 and 5, not yet in RRT (156 had a creatinine clearance <20ml/min/1.73m2 [5 non-dialysis] and 30 had a clearance between 20 and 30ml/min/1.73m2), with no impairment of their cognitive abilities and accepting to sign informed consent. Exclusion criteria were: active neoplasia, active infection or severe lung disease. The period of patient's recruitment was from March 2008 to September 2011.
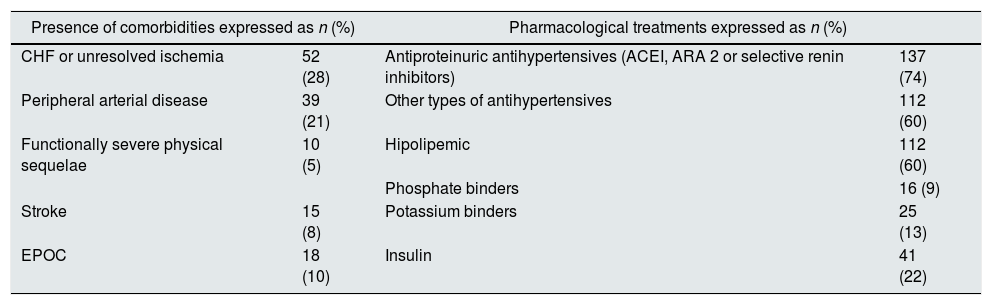
The mean age was 66.15±16.01; a 54.3% (n=101) were males. The etiology of renal disease was: 76 (40.9%) diabetes mellitus (DM), of which, 6 (7.9%) had type 1 DM; 26 (14%) glomerular disease; 24 (13.4%) nephroangiosclerosis; others 19 (14.35%) and not identified in 11 patients (10.3%). Table 1 shows the co-morbidities and pharmacological treatment of the patients there were no differences by gender.
Percentage of co-morbidities and pharmacological treatments of 186 patients in the advanced CKD clinic.
| Presence of comorbidities expressed as n (%) | Pharmacological treatments expressed as n (%) | ||
|---|---|---|---|
| CHF or unresolved ischemia | 52 (28) | Antiproteinuric antihypertensives (ACEI, ARA 2 or selective renin inhibitors) | 137 (74) |
| Peripheral arterial disease | 39 (21) | Other types of antihypertensives | 112 (60) |
| Functionally severe physical sequelae | 10 (5) | Hipolipemic | 112 (60) |
| Phosphate binders | 16 (9) | ||
| Stroke | 15 (8) | Potassium binders | 25 (13) |
| EPOC | 18 (10) | Insulin | 41 (22) |
Out of the 186 patients studied, 76 had DM, 6 of which were DM type I. ARA 2: angiotensin 2 receptor antagonists; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; ACE inhibitors: angiotensin converting enzyme inhibitors.
Clinical information collected included underlying disease, pharmacological treatment, comorbidities or intercurrent processes that could affect nutritional status.
All patients received the dietary instructions from the nephrologist, according to the usual clinical practice and following the K/DOQI guidelines.15
Analytical determinationsFasting blood samples were collected and the following parameters were measured: albumin, prealbumin, creatinine clearance, creatinine, potassium, phosphorus, C-reactive protein (CRP), total lymphocyte count, total cholesterol, LDL, HDL and triglycerides. Measurements in 24h urine collection: urine volume and proteinuria. All measurements were performed by the Biochemistry Laboratory of the Hospital Universitario La Paz following standardized methods. Albumin was measured using the bromocresol green method.
Anthropometric parameters and body compositionMeasurement of anthropometric parameters was adjusted to the standard technique in compliance with the directions recommended by WHO, 1976. These measurements were obtained with the subject barefoot and in underwear. A digital balance was used to measure the body weight (TANITA BC-420MA, Biological Medical Technology S.L. Barcelona, Spain). The height was obtained by a millimeter precision rod (range: 80–200cm). An inextensible metric tape of 0.1cm precision was used to measure arm circumference (AC). The tricipital skinfold thickness (TST) was obtained by means of a Holtain plímeter of 20cm of amplitude and sensitivity of 0.2mm. The body mass index (BMI) (weight in kg/[height in cm]2) was calculated from the anthropometric measurements of weight and height.
Body composition was evaluated in 80 patients with a vector bioimpedance (BIVA) analyzer (Akern Florence, Italy) that measures at 50Hz and at an intensity of 0.8mA. Tetrapolar and total. With a standard error of 2%. The measurement was performed according to the criteria established by the National Institute of Health Technology Assessment Conference Statement.16 If patient had vascular access, all measurements were performed on the contralateral side.17 The following parameters were calculated from the values of resistance and reactance, all in percentage: fat mass (FM), muscle mass (MM), fat free mass (FFM). Body fluids, (all shown in percentage): total body water (TBW), extracellular water (ECW) and intracellular water (ICW), as well as phase angle (AP°), interchange Na/K, cell mass and cell mass index (BCMI).
Food intakeEach patient was asked to document the intake, including fluids, through a 3-day period, one of them of weekend. The caloric and nutritional value of the diet was quantified using the nutritional program DietSOURCE® 3.0 (Consumer Health SA).
Nutritional statusThe evaluation of the nutritional status was performed using SGA and PEW criteria of the ISRNM.
We used the SGA of Detsky,14 which is divided into 5 scales assessing: percentage of weight loss in the last 3–6 months, changes in dietary intake, intestinal symptoms during the last 2 weeks (nausea, vomiting, diarrhea, anorexia) and the presence of any functional disability. The fourth scale is a physical examination that evaluates the loss of FM or MM, the presence of edema and ascites, and parameter received a score according to normal-mild-moderate-severe.
Once all information was recorded, patients were classified into 3 categories, A (normal nutrition), B (mild or moderately malnourished) and C (malnourished patient).
To determine the nutritional status according to the criteria of ISRNM, it is necessary that the patient meet 3 of the 4 categories that determine the presence of PEW, and had to be reproduced y in at least 2 determinations:
- -
biochemical category: albumin <3.8g/dl; prealbumin <30mg/dl; cholesterol <100mg/dl without hypolipemic medication18 (this was not considered because most patients, n=147, were on medication).
- -
body mass category: BMI<23kg/m2; unintentional weight loss of 5% in the last 3 months or 10% in the last 6 months.
- -
MM category: reduction of 10% of AC relative to p50.
- -
Intake category: protein catabolic rate (nPCR) <0.6g/kg weight/day; energy intake <25kcal/kg weight/day maintained over a period of 2 months. (Actual weight, optimal weight or adjusted weight according to the K/DOQI guidelines have been used for the energy adjustment.)15
Qualitative variables are shown as absolute frequencies and percentages; quantitative variables are presented as mean and the standard deviation (X±SD).
The comparison of the qualitative variables between two or more groups was performed using the chi-square test or Fisher's exact test, depending on the distribution of the data. Comparison of two quantitative variables was performed using the Mann–Whitney U test or the Student t test, depending on the data distribution.
The profile of the patients with PEW, was determine by conditional step-forward multivariate logistic regression analysis. The results of the model adjustment are described as odds ratio (OR), with their corresponding 95% CI, and the p values obtained.
All statistical tests were contemplated as bilateral, with a significance level of 0.05. The statistical analysis was performed using the statistical program SPSS 17.0.
ResultsGeneral description of the patientsAll variables were categorized by gender, no differences were observed between men and women; the mean age of men and women were was 65.4±16.1 and 67.1±16.0 years respectively, with no significant differences.
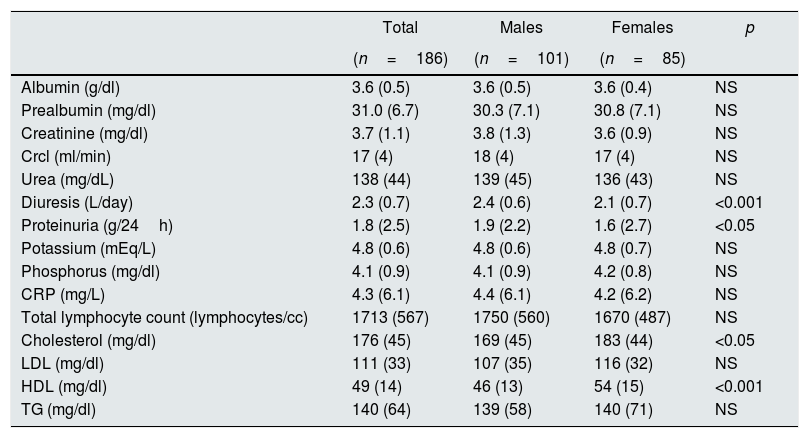
As shown in Table 2, patients had low levels of albumin with normal levels of prealbumin, elevated serum concentrations of CRP and proteinuria. Men had a greater diuresis and proteinuria than women. Values of total cholesterol and HDL were higher in women than men but cholesterol levels did not reach pathological values.
Biochemical parameters in 186 patients with advanced CKD stratified by gender.
| Total | Males | Females | p | |
|---|---|---|---|---|
| (n=186) | (n=101) | (n=85) | ||
| Albumin (g/dl) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.4) | NS |
| Prealbumin (mg/dl) | 31.0 (6.7) | 30.3 (7.1) | 30.8 (7.1) | NS |
| Creatinine (mg/dl) | 3.7 (1.1) | 3.8 (1.3) | 3.6 (0.9) | NS |
| Crcl (ml/min) | 17 (4) | 18 (4) | 17 (4) | NS |
| Urea (mg/dL) | 138 (44) | 139 (45) | 136 (43) | NS |
| Diuresis (L/day) | 2.3 (0.7) | 2.4 (0.6) | 2.1 (0.7) | <0.001 |
| Proteinuria (g/24h) | 1.8 (2.5) | 1.9 (2.2) | 1.6 (2.7) | <0.05 |
| Potassium (mEq/L) | 4.8 (0.6) | 4.8 (0.6) | 4.8 (0.7) | NS |
| Phosphorus (mg/dl) | 4.1 (0.9) | 4.1 (0.9) | 4.2 (0.8) | NS |
| CRP (mg/L) | 4.3 (6.1) | 4.4 (6.1) | 4.2 (6.2) | NS |
| Total lymphocyte count (lymphocytes/cc) | 1713 (567) | 1750 (560) | 1670 (487) | NS |
| Cholesterol (mg/dl) | 176 (45) | 169 (45) | 183 (44) | <0.05 |
| LDL (mg/dl) | 111 (33) | 107 (35) | 116 (32) | NS |
| HDL (mg/dl) | 49 (14) | 46 (13) | 54 (15) | <0.001 |
| TG (mg/dl) | 140 (64) | 139 (58) | 140 (71) | NS |
Values are mean (standard deviation). CrCl: creatinine clearance; HDL: high density lipoprotein; LDL: low density lipoprotein; PCR: C-reactive protein; TG: triglycerides.
Proteinuria was slightly superior but statistically significant in males vs. women. There was a wide range of proteinuria and given its influence on nutritional status, the proteinuria was categorized into the following ranges: ≤0.5g/24h, 0.6–3g/24h and >3g/24h. It was found that proteinuria values > 3g/dl were more frequent in male than females (39 vs. 30%, p <0.05). Proteinuria correlated negatively with serum albumin levels (r=−0.446, p<0.05).
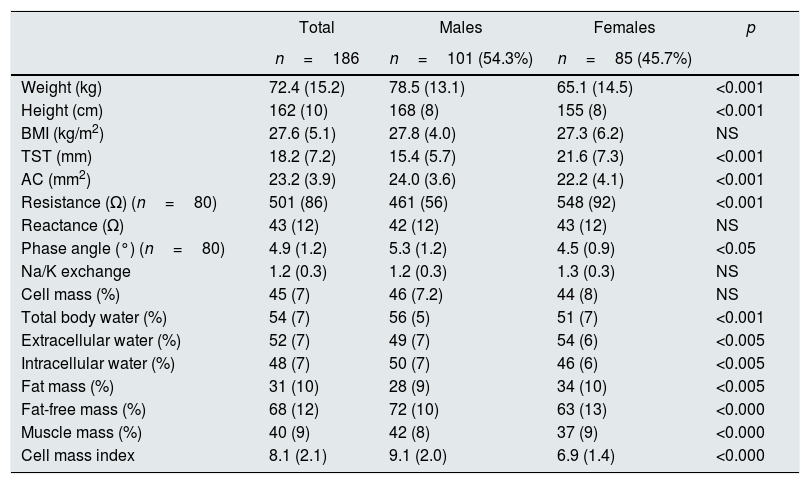
Regarding the anthropometric parameters (Table 3), the mean BMI was 27.0±5.10kg/m2; the patients studied were in the overweight grade II range, with the following distribution of BMI: 4 patients (2.5%) had a BMI <18.5kg/m2; 52 patients were within normal range (BMI: 18.5–24.9kg/m2); the majority, 70 patients, were in the overweight range (BMI: 25–29.9kg/m2); 58 presented obesity (BMI>30kg/m2) and 3 of them had morbid obesity.
Parameters of anthropometry and bioimpedance from the 186 patients included stratified by gender.
| Total | Males | Females | p | |
|---|---|---|---|---|
| n=186 | n=101 (54.3%) | n=85 (45.7%) | ||
| Weight (kg) | 72.4 (15.2) | 78.5 (13.1) | 65.1 (14.5) | <0.001 |
| Height (cm) | 162 (10) | 168 (8) | 155 (8) | <0.001 |
| BMI (kg/m2) | 27.6 (5.1) | 27.8 (4.0) | 27.3 (6.2) | NS |
| TST (mm) | 18.2 (7.2) | 15.4 (5.7) | 21.6 (7.3) | <0.001 |
| AC (mm2) | 23.2 (3.9) | 24.0 (3.6) | 22.2 (4.1) | <0.001 |
| Resistance (Ω) (n=80) | 501 (86) | 461 (56) | 548 (92) | <0.001 |
| Reactance (Ω) | 43 (12) | 42 (12) | 43 (12) | NS |
| Phase angle (°) (n=80) | 4.9 (1.2) | 5.3 (1.2) | 4.5 (0.9) | <0.05 |
| Na/K exchange | 1.2 (0.3) | 1.2 (0.3) | 1.3 (0.3) | NS |
| Cell mass (%) | 45 (7) | 46 (7.2) | 44 (8) | NS |
| Total body water (%) | 54 (7) | 56 (5) | 51 (7) | <0.001 |
| Extracellular water (%) | 52 (7) | 49 (7) | 54 (6) | <0.005 |
| Intracellular water (%) | 48 (7) | 50 (7) | 46 (6) | <0.005 |
| Fat mass (%) | 31 (10) | 28 (9) | 34 (10) | <0.005 |
| Fat-free mass (%) | 68 (12) | 72 (10) | 63 (13) | <0.000 |
| Muscle mass (%) | 40 (9) | 42 (8) | 37 (9) | <0.000 |
| Cell mass index | 8.1 (2.1) | 9.1 (2.0) | 6.9 (1.4) | <0.000 |
Values are mean (standard deviation). AC: arm circumference; BMI: body mass index; TST: tricipital skinfold thickness.
The body composition measured by bioimpedance showed low MM, with normal levels of FM. As far as body fluids, it was found: slightly elevated ECW, with a decrease in ICW, as reflected in the values of Na/K exchange levels with normal TBW. Males had a greater body weight, no difference in BMI, and had high values of AC and more FFM and MM than women, who presented higher values of FM and TST. Males had a higher percentage of TBW, with a slight predominance of ICW, the opposed was found in females. Variables directly related to the nutritional status indicate that women showed a worse nutritional situation with lower values of AP° and of BCMI.
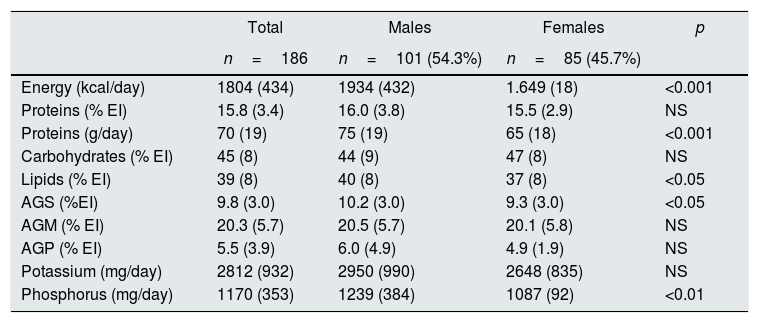
Analysis of the diets (Table 4) showed that both caloric and lipid intakes were within normal ranges; however, important differences were observed between males and females: energy intake was significantly higher in males, accompanied by higher intake of protein, lipid intake, and saturated fatty acids (SFA). Intake of phosphorus and potassium was greater in males than females.
Dietary parameters from the 186 patients included stratified by gender.
| Total | Males | Females | p | |
|---|---|---|---|---|
| n=186 | n=101 (54.3%) | n=85 (45.7%) | ||
| Energy (kcal/day) | 1804 (434) | 1934 (432) | 1.649 (18) | <0.001 |
| Proteins (% EI) | 15.8 (3.4) | 16.0 (3.8) | 15.5 (2.9) | NS |
| Proteins (g/day) | 70 (19) | 75 (19) | 65 (18) | <0.001 |
| Carbohydrates (% EI) | 45 (8) | 44 (9) | 47 (8) | NS |
| Lipids (% EI) | 39 (8) | 40 (8) | 37 (8) | <0.05 |
| AGS (%EI) | 9.8 (3.0) | 10.2 (3.0) | 9.3 (3.0) | <0.05 |
| AGM (% EI) | 20.3 (5.7) | 20.5 (5.7) | 20.1 (5.8) | NS |
| AGP (% EI) | 5.5 (3.9) | 6.0 (4.9) | 4.9 (1.9) | NS |
| Potassium (mg/day) | 2812 (932) | 2950 (990) | 2648 (835) | NS |
| Phosphorus (mg/day) | 1170 (353) | 1239 (384) | 1087 (92) | <0.01 |
Values are mean (standard deviation). MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; EI: energy intake; NS: not significant.
The nutritional status was analyzed by SGA and using PEW criteria. By SGA a 72.1% (134) had a normal nutrition (category A), 27.9% (52) presented some degree of malnutrition, 25.8% (48) corresponded to category B and only 2.2%4 presented severe malnutrition (category C). No differences by gender were found in any of the categories.
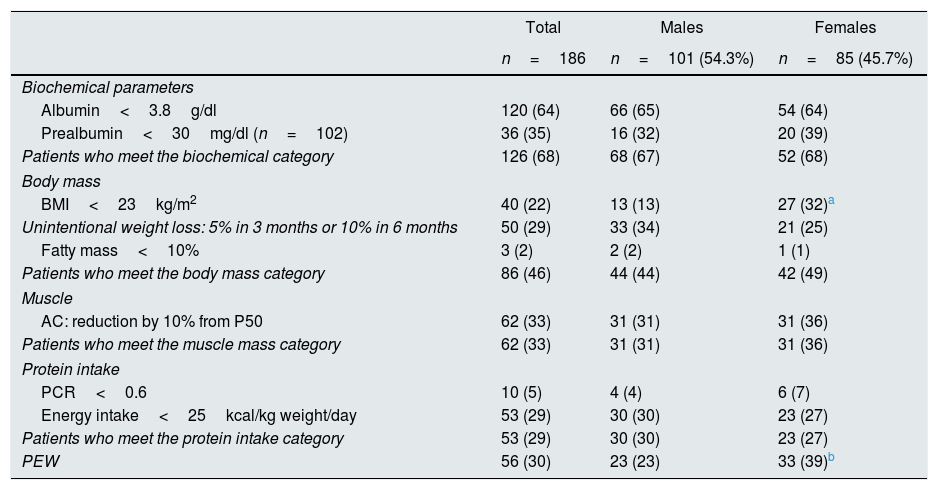
According to the ISRNM criteria, 56 (30.10%) of patients had PEW (Table 5), with significant differences by gender (women had more PEW than men). There were no differences by categories, and the BMI<23kg/m2 was the only criterion that was more frequent in women than men (31.8 vs. 12.9%, p<0.01).
Criteria for PEW in 186 patients studied, total and categorized by gender. Values are expressed as n (%).
| Total | Males | Females | |
|---|---|---|---|
| n=186 | n=101 (54.3%) | n=85 (45.7%) | |
| Biochemical parameters | |||
| Albumin<3.8g/dl | 120 (64) | 66 (65) | 54 (64) |
| Prealbumin<30mg/dl (n=102) | 36 (35) | 16 (32) | 20 (39) |
| Patients who meet the biochemical category | 126 (68) | 68 (67) | 52 (68) |
| Body mass | |||
| BMI<23kg/m2 | 40 (22) | 13 (13) | 27 (32)a |
| Unintentional weight loss: 5% in 3 months or 10% in 6 months | 50 (29) | 33 (34) | 21 (25) |
| Fatty mass<10% | 3 (2) | 2 (2) | 1 (1) |
| Patients who meet the body mass category | 86 (46) | 44 (44) | 42 (49) |
| Muscle | |||
| AC: reduction by 10% from P50 | 62 (33) | 31 (31) | 31 (36) |
| Patients who meet the muscle mass category | 62 (33) | 31 (31) | 31 (36) |
| Protein intake | |||
| PCR<0.6 | 10 (5) | 4 (4) | 6 (7) |
| Energy intake<25kcal/kg weight/day | 53 (29) | 30 (30) | 23 (27) |
| Patients who meet the protein intake category | 53 (29) | 30 (30) | 23 (27) |
| PEW | 56 (30) | 23 (23) | 33 (39)b |
Values shown as percentages.
AC: arm muscle circumference; PEW: protein energy wasting; BMI: body mass index; PCR: protein catabolic rate; P50: fifty percentile.
A 30.1% of the patients showed PEW, and they presented a higher proportion of functionally severe physical sequelae (12.5 vs. 1.5%, p<0.001); however, there were no differences in the rest of comorbidities, or in the pharmacological treatment.
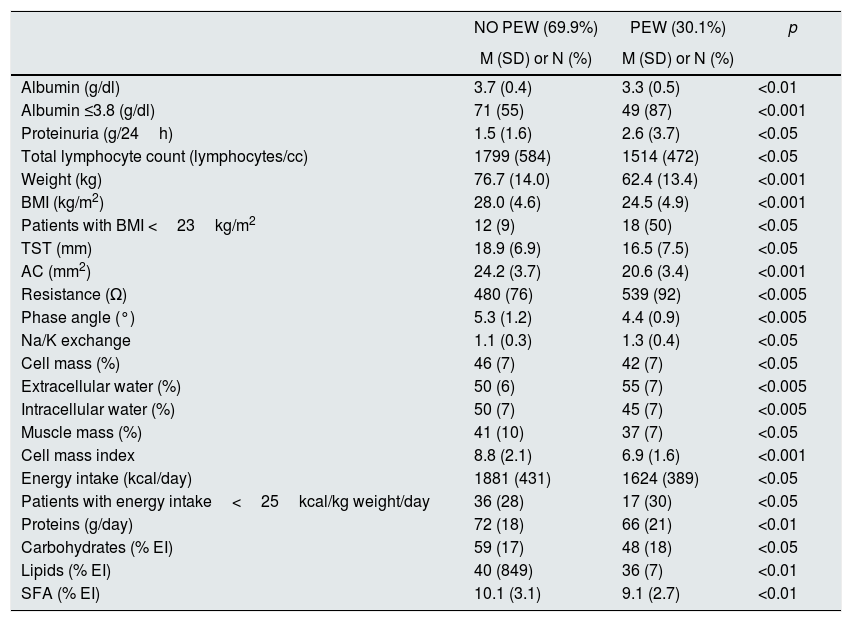
Table 6 shows the variables that were significantly different in patients according to the presence or absence of PEW.
Biochemical, anthropometric, body composition and dietary parameters according to the presence or absence of PEW.
| NO PEW (69.9%) | PEW (30.1%) | p | |
|---|---|---|---|
| M (SD) or N (%) | M (SD) or N (%) | ||
| Albumin (g/dl) | 3.7 (0.4) | 3.3 (0.5) | <0.01 |
| Albumin ≤3.8 (g/dl) | 71 (55) | 49 (87) | <0.001 |
| Proteinuria (g/24h) | 1.5 (1.6) | 2.6 (3.7) | <0.05 |
| Total lymphocyte count (lymphocytes/cc) | 1799 (584) | 1514 (472) | <0.05 |
| Weight (kg) | 76.7 (14.0) | 62.4 (13.4) | <0.001 |
| BMI (kg/m2) | 28.0 (4.6) | 24.5 (4.9) | <0.001 |
| Patients with BMI <23kg/m2 | 12 (9) | 18 (50) | <0.05 |
| TST (mm) | 18.9 (6.9) | 16.5 (7.5) | <0.05 |
| AC (mm2) | 24.2 (3.7) | 20.6 (3.4) | <0.001 |
| Resistance (Ω) | 480 (76) | 539 (92) | <0.005 |
| Phase angle (°) | 5.3 (1.2) | 4.4 (0.9) | <0.005 |
| Na/K exchange | 1.1 (0.3) | 1.3 (0.4) | <0.05 |
| Cell mass (%) | 46 (7) | 42 (7) | <0.05 |
| Extracellular water (%) | 50 (6) | 55 (7) | <0.005 |
| Intracellular water (%) | 50 (7) | 45 (7) | <0.005 |
| Muscle mass (%) | 41 (10) | 37 (7) | <0.05 |
| Cell mass index | 8.8 (2.1) | 6.9 (1.6) | <0.001 |
| Energy intake (kcal/day) | 1881 (431) | 1624 (389) | <0.05 |
| Patients with energy intake<25kcal/kg weight/day | 36 (28) | 17 (30) | <0.05 |
| Proteins (g/day) | 72 (18) | 66 (21) | <0.01 |
| Carbohydrates (% EI) | 59 (17) | 48 (18) | <0.05 |
| Lipids (% EI) | 40 (849) | 36 (7) | <0.01 |
| SFA (% EI) | 10.1 (3.1) | 9.1 (2.7) | <0.01 |
Values shown as mean (standard deviation), except for non-quantitative variables that are shown as n (%).
SFA: saturated fatty acids; AC: arm circumference; PEW: protein energy wasting; EI: energy intake; BMI: muscle mass index; TST: tricipital skinfold thickness.
Patients with PEW presented lower values of albumin and TLC, and greater proteinuria than patients without PEW; no differences were observed in the rest of parameters.
Regarding anthropometric and bioimpedance parameters, patients with PEW were characterized by lower body weight, lower BMI and in terms of body composition, they had lower TST, AC and MM. The TBW was very similar in the two groups, although their distribution was different: patients with PEW presented higher levels of ECW, with lower ICW. The AP°, cell mass and BCMI were also lower in the PEW group.
Analysis of the dietary habits showed that, the energy intake and the consumption of macronutrients were significantly reduced in the PEW group: protein, percentage of CH and lipids; however, despite the lower consumption of monounsaturated fatty acids (MUFAs) (19.3±5.1 vs. 20.8±5.9; p=ns) and polyunsaturated fatty acids (PUFAs) (5.1±0.12 vs. 5.8±4.5, p=ns). 5.7±4.5, p=ns, only the SFA showed significant difference. The PEW group had a lower intake of minerals that did not reach significance: potassium (2661±106 vs. 2876±868mg/dia) and phosphorus (1101±326 vs. 1199±360mg/dl).
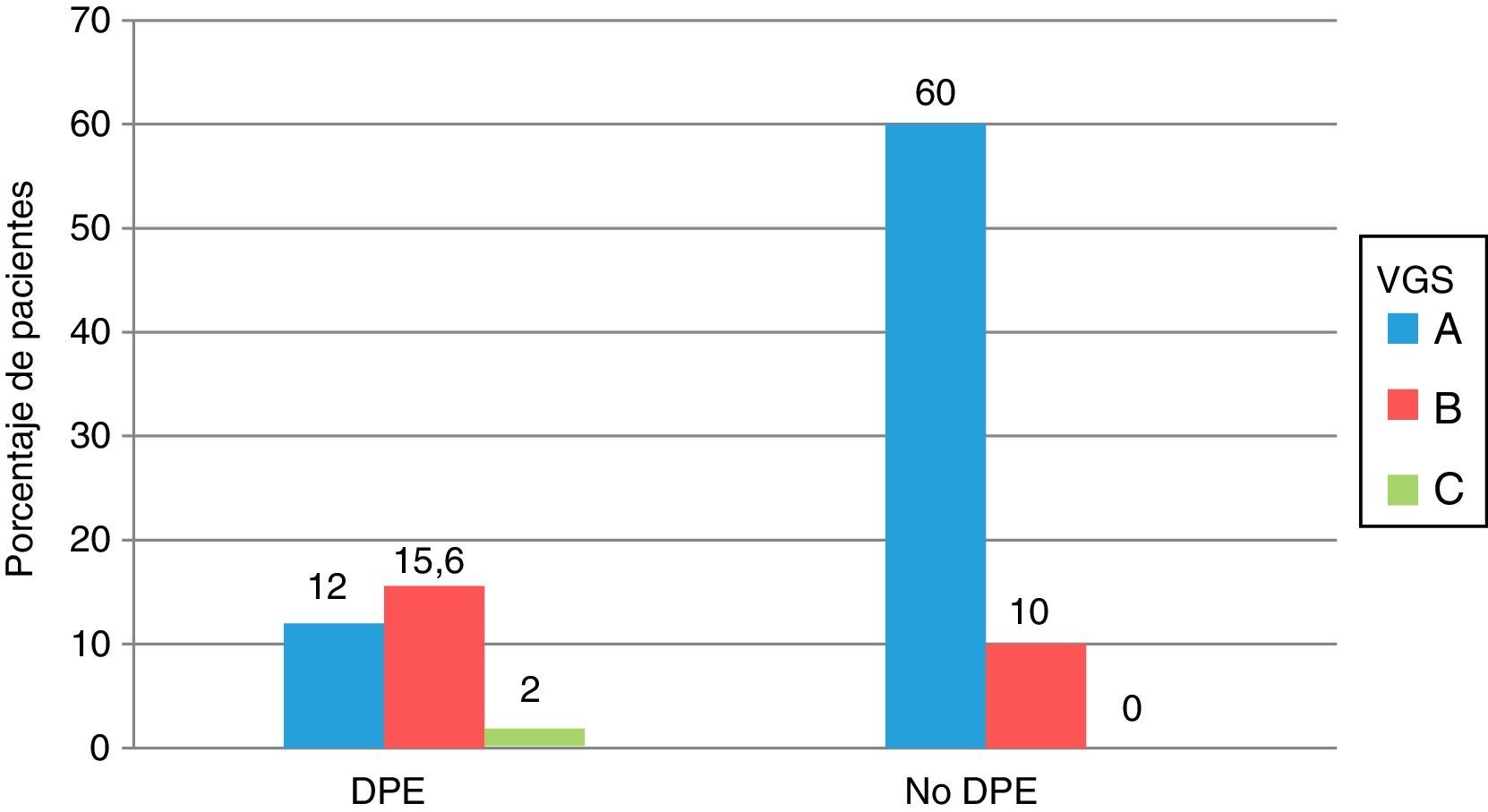
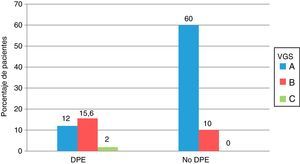
Comparison of the nutritional status according to SGA and by PEW criteria is shown in Fig. 1. Seventeen of the 56 patients with PEW had a normal nutrition according to SGA (category A) and 19 of the 130 patients without PEW were moderately malnourished (category B), although the differences were not significant.
Comparison of the nutritional status of the 186 patients with advanced CKD, assessed according to the criteria of protein energy attrition of the ISRMN and by subjective global assessment. All values are expressed as percentages. PEW: protein energy wasting; SGA: subjective global assessment.
The ISRNM criteria with respect to SGA have a sensitivity of 82.2% (95% CI: 75.6–88.3), and specificity of 62% (95% CI: 48.2–74.1) and an OR for diagnosis of 7.87 (95% CI: 3.81–16.28).
Regarding the criteria that define PEW, we found significant differences in albumin, BMI and energy intake, however there were no differences in the different categories among themselves.
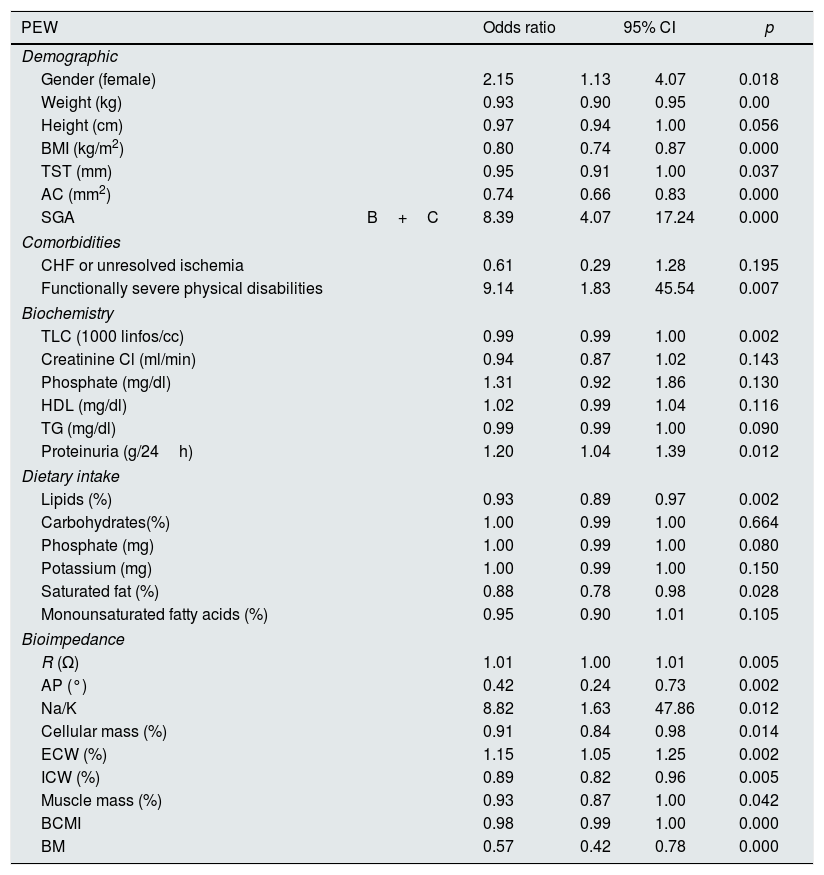
Tables 7 and 8 show the results of the univariate and multivariate logistic regression analysis.
Univariate regression model. Odds ratios and 95% confidence intervals for the presence of protein energy wasting in 186 patients with advanced CKD.
| PEW | Odds ratio | 95% CI | p | ||
|---|---|---|---|---|---|
| Demographic | |||||
| Gender (female) | 2.15 | 1.13 | 4.07 | 0.018 | |
| Weight (kg) | 0.93 | 0.90 | 0.95 | 0.00 | |
| Height (cm) | 0.97 | 0.94 | 1.00 | 0.056 | |
| BMI (kg/m2) | 0.80 | 0.74 | 0.87 | 0.000 | |
| TST (mm) | 0.95 | 0.91 | 1.00 | 0.037 | |
| AC (mm2) | 0.74 | 0.66 | 0.83 | 0.000 | |
| SGA | B+C | 8.39 | 4.07 | 17.24 | 0.000 |
| Comorbidities | |||||
| CHF or unresolved ischemia | 0.61 | 0.29 | 1.28 | 0.195 | |
| Functionally severe physical disabilities | 9.14 | 1.83 | 45.54 | 0.007 | |
| Biochemistry | |||||
| TLC (1000 linfos/cc) | 0.99 | 0.99 | 1.00 | 0.002 | |
| Creatinine Cl (ml/min) | 0.94 | 0.87 | 1.02 | 0.143 | |
| Phosphate (mg/dl) | 1.31 | 0.92 | 1.86 | 0.130 | |
| HDL (mg/dl) | 1.02 | 0.99 | 1.04 | 0.116 | |
| TG (mg/dl) | 0.99 | 0.99 | 1.00 | 0.090 | |
| Proteinuria (g/24h) | 1.20 | 1.04 | 1.39 | 0.012 | |
| Dietary intake | |||||
| Lipids (%) | 0.93 | 0.89 | 0.97 | 0.002 | |
| Carbohydrates(%) | 1.00 | 0.99 | 1.00 | 0.664 | |
| Phosphate (mg) | 1.00 | 0.99 | 1.00 | 0.080 | |
| Potassium (mg) | 1.00 | 0.99 | 1.00 | 0.150 | |
| Saturated fat (%) | 0.88 | 0.78 | 0.98 | 0.028 | |
| Monounsaturated fatty acids (%) | 0.95 | 0.90 | 1.01 | 0.105 | |
| Bioimpedance | |||||
| R (Ω) | 1.01 | 1.00 | 1.01 | 0.005 | |
| AP (°) | 0.42 | 0.24 | 0.73 | 0.002 | |
| Na/K | 8.82 | 1.63 | 47.86 | 0.012 | |
| Cellular mass (%) | 0.91 | 0.84 | 0.98 | 0.014 | |
| ECW (%) | 1.15 | 1.05 | 1.25 | 0.002 | |
| ICW (%) | 0.89 | 0.82 | 0.96 | 0.005 | |
| Muscle mass (%) | 0.93 | 0.87 | 1.00 | 0.042 | |
| BCMI | 0.98 | 0.99 | 1.00 | 0.000 | |
| BM | 0.57 | 0.42 | 0.78 | 0.000 | |
ECW: extracellular water; AF: phase angle; ICW: intracellular water; BCMI: cell mass index; AC: arm circumference; CHF: congestive heart failure; BMI: body mass index; BM: basal metabolism; TST: tricipital skinfold thickness; R: resistance; TLC: total lymphocyte count; TG: triglycerides; SGA: subjective global assessment.
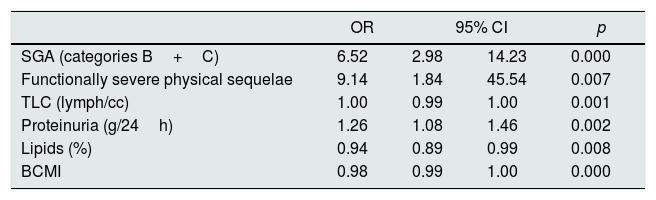
Multivariate regression model. Odds ratios and 95% confidence intervals for the presence of protein energy wasting in 186 patients with advanced CKD.
| OR | 95% CI | p | ||
|---|---|---|---|---|
| SGA (categories B+C) | 6.52 | 2.98 | 14.23 | 0.000 |
| Functionally severe physical sequelae | 9.14 | 1.84 | 45.54 | 0.007 |
| TLC (lymph/cc) | 1.00 | 0.99 | 1.00 | 0.001 |
| Proteinuria (g/24h) | 1.26 | 1.08 | 1.46 | 0.002 |
| Lipids (%) | 0.94 | 0.89 | 0.99 | 0.008 |
| BCMI | 0.98 | 0.99 | 1.00 | 0.000 |
BCMI: cell mass index; TLC: total lymphocyte count; SGA: subjective global assessment.
This study reveals the presence of malnutrition in Spanish patients with advanced CKD. Different nutritional assessment tools assessed the nutritional state. The study also identifies the main factors associated with PEW. The multivariate analysis showed that TLC, proteinuria, percentage of lipid intake, BCMI and the presence of functionally severe physical sequelae are variables that predict PEW in this patient population. None of these variables is included in the classical definition of PEW, but it is related with each of its categories.
Comparison of epidemiological aspectsThe prevalence of malnutrition in our patients is 30.1% by ISRNM criteria and by SGA it is 27.9%. These values are higher than those reported by other authors in different non-European populations. In Australian patients, Campbell et al.19 evaluated 56 patients and observed prevalence of malnutrition in 12% patients and Lawson et al.,11 using the same tools observed a 28% in 122 patients. Cuppari et al.,20 using SGA, found that 11% of the 922 patients studied had malnutrition, and a 32% were at risk of malnutrition; however, other Brazilian groups have not found malnutrition in their patients using anthropometric, biochemical and intake parameters.21,22 The recent study by Amparo et al.23 found values higher than ours in the 300 patients with advanced CKD, a 63.7% had malnutrition inflammation scale (MIS)>3 and a 19% MIS >8.
In the European population, the prevalence of malnutrition observed in these patients has been also lower than in our study. Kovesdy et al.13 studied 1220 patients and reported that 44.8% presented albumin levels below 3.7mg/dl, this is lower than our values; if the criterion is of albumin concentration <3.8mg/dl, we found figures of 68.8%. Westland et al.24 found percentages of malnutrition in the Norwegian population of 11% measured by SGA. Therefore the data on prevalence of malnutrition, varies according to the method used and on the stage of the renal disease, but we, as other authors2,25 also find that geographic and cultural factors also determine its occurrence.
Incidence of malnutrition in advanced chronic kidney disease and dialysis (renal replacement therapy). Sequential relationshipThe presence of malnutrition in RRT patients in Spain resulted to be high if the assessment is based only on biochemical parameters. Yuste et al.26 studied the prevalence of malnutrition during one year of follow-up in 124 patients on hemodialysis, and found malnutrition in 71.9% when using albumin values <3.8mg/dl; 64.6% if the criterion was prealbumin <30mg/dl and only 3.4% if it was based on total cholesterol <100mg/dl. Ruperto et al.27 unified the criteria of malnutrition by the sum of albumin, arm circumference (AC) and body weight, and found malnutrition in 52.5% of the 80 patients studied. In peritoneal dialysis, published studies indicate that the incidence of malnutrition is 21%.28 In advanced CKD, the data indicate that the incidence of malnutrition increases as the renal function deteriorates, and reaches its maximum when dialysis becomes necessary. There is a temporal projection from the advanced stage of CKD to dialysis, so that the knowledge of the nutritional state and its treatment could avoid a state of malnutrition at the commencement of dialysis which entails difficulty of treatment and a worse prognosis.29
Elements that complete the definition of nutritional status in our patients with advanced chronic kidney diseaseWe evaluated the nutritional status using the ISRNM criteria and common parameters of daily clinical practice in the advanced CKD clinic. We found that some of these parameters could help to identify malnourished patients in our advanced CKD population. Proteinuria>10g/24h is considered to be one of the causes of PEW30 however, we found that PEW is found in patients with much lower proteinuria (≥2.5g/24h). TLC is one of the classic markers of poor nutritional status31 and mortality in patients with advanced CKD.13 This was confirmed with our data.
The role of monofrequency bioimpedance as a nutritional marker has been established already.32,33 In our study, most of the evaluated variables of body composition confirmed the presence of malnutrition characterized by low deposits of FM, MM, inverted ECW/ICW ratio, lower levels of BCMI and low AP; all these parameters are different in males and female.34 The panel of experts of the ISRNM, proposes that bioimpedance parameters and biochemical parameters such as the percentage of lymphocytes1 could be used as additional criteria to define PEW. The data found in our population confirm the appropriateness of including these parameters for the diagnosis of PEW in advanced CKD.
Despite the limitations of BMI calculation which varies with: age, gender, hydration status in CKD patients, and is not able distinguish FM from lean mass,35 we considered that its use is adequate in our patients since most of them presented an adequate state of hydration as reflected by the mean percent of TBW 54.3±6.7.
Prealbumin values are increased in dialysis patients,36 but the cut-off point has not been established in the advanced CKD population. A 35.3% presented prealbumin values <30mg/dl, without differences by groups. As reported by other authors, we consider that prealbumin is not a definitive marker of PEW, and that it would be necessary to use it together with other markers for the assessment of nutritional status in patients with advanced CKD.37
Despite the recognized relationship between malnutrition and inflammation,38 we have not found such a relationship in this study which may be due to the low degree of inflammation observed in our patients which presented an average CRP of 4.3±6.1mg/l.
Protein energy wasting modifies the diagnosis of malnutrition done by subjective global assessmentThe SGA in its different modalities has been the method most frequently used to make the diagnosis of malnutrition; this is due to the fact that a relationship of SGA with nutritional markers and mortality in patients with advanced CKD has been demonstrated.39 In our study, we did not find differences in the diagnosis of malnutrition performed by ISRNM and SGA criteria, but we considered that it would be convenient to use some objective measurements, such as body composition, for a more complete assessment.40 Despite the small differences found in the diagnosis of nutritional status, PEW (30.1%) vs. SGA (27.9%); we believe that the state of persistent inflammation, proteinuria and the scale used for SGA – in this case the classical Detsky,14 where clinical practice plays a key role – may have contributed to these results.
Of the 4 diagnostic categories of PEW, the majority of our patients match the biochemistry and the one that do not match is the intake, with the characteristic that only 5.4% of patients have low protein intake. In our opinion, this may be due to the difficulty to a low protein intake, and we consider that more energy intake, based on a lipid diet plays a conservative role.
In summary, our data indicates that nutritional tools should be validated according to the stage of disease,23 the geographical area2,7 and other factors such as proteinuria. Assessment of body composition is important and it the dynamic nature of malnutrition reflected in the PEW criteria should be taken into consideration.1,2
The main limitation of our study is that not all variables were evaluated in all subjects, such as bioimpedance (n=80), prealbumin (n=102) and the inclusion of parameters of muscle strength, as well as not being able to show change in dynamic variables of PEW (the loss of muscle mass) or physical activity.
In conclusion, our study shows that some Spanish patients with advanced CKD have PEW as assessed by different tools. The percentage of PEW is higher than that reported in other countries. We believe that it is convenient to consider new diagnostic elements to achieve better nutritional assessment, as well as to adapt the criteria of PEW, not only to the stage of the disease, but also to the geographical area; and, both proteinuria and BCMI are key variables to monitor nutritional status in patients with advanced CKD.
Conflicts of interestThe authors declare that they have no potential conflicts of interest related to the contents of the article.
To Nutrición Médica, who sponsored the study providing salary support for one researcher of the Nutrition Unit team.
To Dr. Cigarrán for his support and motivation in the realization of the article.
Please cite this article as: Pérez-Torres A, González Garcia ME, San José-Valiente B, Bajo Rubio MA, Celadilla Diez O, López-Sobaler AM, et al. Síndrome de desgaste proteico energético en la enfermedad renal crónica avanzada: prevalencia y características clínicas específicas. Nefrologia. 2018;38:141–151.