To the Editor:
The prevalence of hepatitis C virus infection among patients on haemodialysis is estimated at 3%-23% in developed countries. Some of the adverse effects described in treating patients on haemodialysis with interferon as a monotherapy include1: flu-like symptoms, anaemia, immunological graft intolerance, depression, leukopoenia, confusion, diarrhoea, bone pain, thyroid alterations, thrombocytopenia, and convulsions. To a lesser degree, there have also been descriptions in the medical literature of autoimmune diseases and neurological alterations in relation to this type of treatment in patients on haemodialysis. Pegylated interferon improves drug absorption and extends the half-life of this medication.
Here we describe the case of a 47-year old male on haemodialysis with no known allergies, a history of smoking and social drinking, and a previous addiction to parenteral drugs. The patient suffered from stage 5 chronic kidney disease of an unknown origin. The patient had received a transplant but had to return to haemodialysis after 8 years. The patient also had arterial hypertension, positive serology for hepatitis C virus (HCV), positive CRP (genotype 3a), positive cryoglobulins, chronic gastritis, bronchiectasis, and had problems with his vascular access in the form of a permanent right jugular catheter.
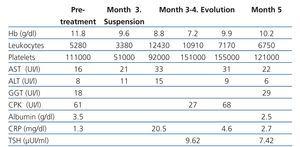
The patient had no alterations in transaminase levels or clotting factors, and in the absence of ultrasound indications of portal hypertension, we started him on treatment with alpha-2-a pegylated interferon as a monotherapy, considering the patient to be a candidate for a second kidney transplant. During the first three months of treatment, the patient suffered only mild adverse side effects related to treatment, in the form of mild thrombocytopenia and nausea (table 1).
After this initial period, a series of complications began to arise:
- Right basal pneumonia with haemoptysis, pleural effusion, and marked thrombocytopenia. At this point we suspended treatment with interferon.
- Epigastric pain with irradiation into the hypochondria and lumbar area that intensified during haemodialysis and that did not recede with analgesia, prompting several incomplete haemodialysis sessions.
- Deterioration in general state of health, including weight loss, anaemia despite increased doses of darbepoetin, and weakness in the legs, prompting hospitalisation. We performed a gastroscopy, observing antral and ectatic gastropathy with no signs of acute bleeding. A computed axial tomography (CAT scan) of the chest detected a solid hilar tumour in the lower right quadrant that was producing a total obstruction of the bronchus in the right lower lobule. We performed a bronchoscopy, extracting the blood clot from the aforementioned location. A bronchial swab and biopsy revealed negative test results. We diagnosed the patient with hypothyroidism.
- Diplopia, inability to close the left eye, and inability to walk, three weeks after suspending interferon and in an outpatient setting. A cranial CAT scan revealed no signs of acute damage, but signs of vascular leukoencephalopathy were observed, which were later confirmed by nuclear magnetic resonance (NMR). A CAT scan of the eye sockets revealed a symmetrical increase in the size of the extra-ocular muscles and the inferior, medial, and superior rectus muscles of both eyes, suggesting thyroid orbitopathy. The patient refused transferral to the neurology department. After an initial neurological examination including electroneurography and NMR, the diagnostic conclusion was that of peripheral nervous system involvement with symptoms of thick fibre sensory neuropathy (deep sensitivity), including paralysis of the left sixth cranial nerve, in relation to treatment with interferon, with spontaneous recovery 3 months after suspending treatment.
We started treating the patient with hormone replacement therapy using levothyroxine. We also considered administering corticosteroids to treat the 6th cranial nerve paralysis, as well as the possibility of an autoimmune component (table 2). However, we decided against this strategy in order to avoid the potential for viral replication (viral RNA had become negative after three months of treatment) and in light of the clinical deterioration of the patient, maintaining a conservative approach. The patient’s symptoms progressively improved after treatment was suspended.
Autoimmune and neurological involvement in patients on haemodialysis treated with pegylated interferon is an uncommon complication.1-3 Measurements of auto-antibodies prior to treatment with interferon is a fundamental step for the differential diagnosis of autoimmune complications. Some authors have described an increase in endogenous interferon-alpha during haemodialysis sessions in patients with positive HCV serology (without treatment), which could explain the decrease in viral load produced during treatment.4 However, this supposed increase in interferon could produce a paradoxical effect, as in our case, and activate the complement system, which could in turn be related to the intra-dialytic lumbar pain.
Paralysis of the 6th cranial nerve is not a common occurrence in the context of thyroid orbitopathy.5 In our case, involvement of the peripheral nervous system and the normal aspect of the external extra-ocular rectus muscle leads us to believe that the peripheral nervous system involvement was the cause of the squinting. Several cases have been published of patients not on haemodialysis who were treated with pegylated interferon and suffered paralysis of the 6th cranial nerve. The induction mechanism for the involvement of the 6th cranial nerve stemming from treatment with interferon is still not understood. The treatment option for many of these cases described was corticosteroids.6-8
Our objective in presenting this case was not to alarm the medical community regarding the complications inherent to the treatment of patients on haemodialysis with interferon, but rather to alert the readership as to the less common and late adverse effects that the nephrologist might be the first to notice.
Conflicts of interest
The authors state that they have no potential conflicts of interest related to the content of this article.
Table 1. Laboratory analysis results
Table 2. Autoimmune state following suspension of interferon