Antecedentes: El embarazo en las pacientes con injerto renal se considera de alto riesgo. Objetivos: Identificar las complicaciones perinatales en las mujeres con trasplante renal atendidas en nuestra institución y compararlas con los reportes de la literatura nacional e internacional. Material y métodos: Se estudiaron las complicaciones perinatales de 18 pacientes con trasplante renal de las que se obtuvieron 19 recién nacidos, atendidas entre el 1 de enero de 2009 y el 31 de diciembre de 2010. Los resultados se compararon con reportes previos. Resultados: Edad materna: 28,27 ± 4,70 años, paridad: 2, intervalo del trasplante a la concepción: 7,52 ± 6,20 años, primera consulta prenatal a las 14,35 ± 6,74 semanas, seguimiento prenatal: 18,88 ± 9,18 semanas, 6 consultas prenatales, edad gestacional al nacimiento: 33,11 ± 8,72 semanas. Complicaciones maternas: operación cesárea: 88,88%, transfusión: 38,88%, anemia: 33,33%, ruptura prematura de membranas: 22,22%, parto pretérmino: 22,22%, infección del tracto urinario: 16,66%, preeclampsia: 11,11%, hipertensión arterial descontrolada: 11,11%, aborto: 11,11%, atonía uterina: 5,55%, diabetes gestacional: 0% y mortalidad: 0%. Complicaciones fetales: prematuridad: 52,63%, mortalidad: 21,05%, cuidados intensivos: 21,05% y bajo peso neonatal por restricción del crecimiento: 10,52%. Complicaciones del trasplante: deterioro de la filtración sin necesidad de diálisis: 5,55%, rechazo: 0% y pérdida del injerto: 0%. Conclusiones: Comparativamente, la frecuencia de las complicaciones perinatales fue elevada. El embarazo no tuvo efecto adverso sobre la función renal ni la supervivencia de las pacientes. El injerto renal estable en mujeres con edad fértil no es necesariamente una contraindicación para el embarazo.
Background: Pregnancy in patients with kidney grafts is considered high-risk. Objectives: Determine perinatal complications in women with kidney transplants treated by our hospital and compare them with complications reported in national and international literature. Material and Method: We studied perinatal complications in 18 patients with renal transplantation who delivered 19 newborns and were treated between 1 January 2009 and 31 December 2010. Results were compared with previous reports. Results: Maternal age: 28.27±4.70 years old, parity: 2, interval from transplant to conception: 7.52±6.20 years, first prenatal visit 14.35±6.74 weeks, prenatal care: 18.88±9.18 weeks, 6 prenatal visits, gestational age at birth: 33.11±8.72 weeks. Maternal complications: cesarean section: 88.88%, blood transfusion: 38.88%, anaemia: 33.33%, premature rupture of membranes: 22.22%, preterm delivery: 22.22%, urinary tract infection: 16.66%, preeclampsia: 11.11%, uncontrolled hypertension: 11.11%, miscarriage: 11.11%, uterine antony: 5.55%, gestational diabetes: 0%, and mortality: 0%. Foetal complications: premature birth: 52.63%, mortality: 21.05%, intensive care: 21.05%, and low birth weight due to growth restriction: 10.52%. Transplantation complications: filtration impairment without need for dialysis: 5.55%, graft rejection: 0%, and graft loss: 0%. Conclusions: The frequency of perinatal complications was high. Pregnancy had no adverse effect on renal function and patient survival. Stable renal grafts in women of childbearing age is not necessarily a contraindication for pregnancy.
INTRODUCTION
Women of childbearing age with Terminal Chronic Renal Failure (TCRF) have a higher frequency of infertility and endocrine conditions that are detrimental to conception.1-4 Successful renal grafting may correct these factors and improve the chance of pregnancy.5 The first case of Renal transplant (Tx) and pregnancy was published in 1963 by Murray et al.6 More recently, in 1976, Davison et al7 proposed the first advisory guidelines for planned pregnancy in this type of illness, which have been modified.8-10
Current recommendations include a waiting period of at least one year for recipients of a kidney from a Living Related Donor (LRD) and two years in the case of a Living Non-Related Donor (LNRD), serum creatinine (Cr) <177mmol/l (<2mg/dl), preferably <133mmol/l (<1.5mg/dl), with no recent episode of acute rejection nor evidence of ongoing rejection, normal blood pressure or with minimal hypertensive treatment (a single drug), absence or minimal proteinuria (<0.5g/day), normal renal ultrasound without pyelocaliceal dilatation, low dose consumption of immunosuppressive drugs (prednisone <15mg/day, azathioprine <2mg/kg/day, cyclosporine <4mg/kg/day, tacrolimus at a therapeutic dose), withdrawal of mycophenolate mofetil and sirolimus before conception, close prenatal monitoring and periodic tests of blood concentration of immunosuppressive drugs to adjust the dose based on physiological changes due to gestation and graft function.9-12
The recommendations of international institutions, such as the US Food and Drug Administration (FDA) and the use of drugs at low doses and the introduction of new medications have helped to reduce the frequency of foetal complications at birth10-14 or congenital malformation, but not maternal complications.15-18 Data from the US National Transplantation Pregnancy Registry (NTPR),19 the UK’s Transplant and Pregnancy Registry20 as well as reports from various countries, such as that by Cruz et al from Mexico,21 Sgro et al from Canada,22 Oliveira et al from Brasil,23 Kurata et al from Japan24 and Fernández et al from Spain,25 have contributed to understanding of the issue. The treatment of maternal, foetal and Tx complications should be individualised, early and involve a multidisciplinary team19-26 including, after delivery care, some form of contraceptive to prevent subsequent pregnancies.27
The aim of this study was to identify the perinatal complications in renal Tx women treated by our hospital staff and compare them with the reports in national and international literature.
MATERIAL AND METHODS
The study comprised 18 pregnant women who had undergone renal Tx and gave birth to 19 children, between 1st January 2009 and 31st December 2010, at the Gynaecology and Obstetrics hospital No. 3 of the Mexican Institute of Social Security in Mexico City, a tertiary care centre for patients with high-risk pregnancies. Through the examination of the patient files the principal data on the mothers, foetuses, renal grafting and perinatal complications were registered.
The maternal data included age, parity, gestational age at first visit, duration of prenatal monitoring, gestational age at birth, number of prenatal visits, hospital admissions, medication during pregnancy, causes for delivery care, type of birth (vaginal, hysterotomy, caesarean section) and the method of postnatal contraception.
The foetal data included number of products, weight, Apgar score at one and five minutes after birth, neonatal age evaluated according to the Capurro method, and survival rate.
The renal Tx data included cause of TCRF, type of donation (LRD, LNRD or cadaveric donor [CD]), time from Tx to conception and time from Tx to birth, dose and modifications to immunosuppressive medication, frequency of systemic arterial hypertension (SAH) and abnormal proteinuria (>300mg/day) and 24h prenatal enuresis. To estimate the Tx filtration the endogenous Cr clearance (ECrC) was calculated by means of age, weight and Cr value, using the Cockroft-Gault formula ([140 – age (years) x weight (kg) / Cr x 72] 0.85). The ECrC prior to delivery care was compared to that from the first prenatal visit.
Perinatal complications were studied as maternal, foetal and renal Tx complications. Maternal complications included miscarriage, caesarean section, premature rupture of membranes (PROM), preterm birth (PB), anaemia, transfusion, preeclampsia-eclampsia (P-E), SAH instability, gestational diabetes, urinary tract infection, birth complication and maternal death. Foetal complications included foetal growth restriction (FGR), acute foetal distress (AFD), chronic foetal distress (CFD), respiratory failure, admission to the Neonatal Intensive Care Unit (NICU), prematurity (<37 weeks), congenital malformations, and death. Renal Tx complications included deterioration in filtration (ECrC reduction), proteinuria increase, episodes of rejection, necessity of biopsy, anti-rejection treatment where applicable, graft loss, and need for dialysis.
Statistics analysis
We used central tendency measures (mean and median), dispersion measures (range, standard deviation) and the Student's t test. A value of P <.05 was considered statistically significant.
RESULTS
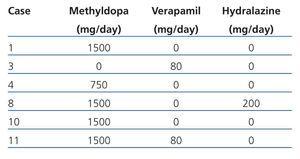
Maternal data: Mean age was 28.27±4.70 years (range 20 to 36), parity (median): 2 (range 1 to 3), gestational age at first prenatal visit: 14.35±6.74 weeks (range 5 to 25), duration of prenatal monitoring: 18.88±9.18 weeks (range 0 to 33), gestational age at birth: 33.11±8.72 weeks (range 8 to 39), prenatal visits (median): 6 (range 1 to 11), hospital admissions (median): 2 (range 1 to 3), vaginal miscarriage: 11.12% (2 cases), caesarean section: 88.88% (16 cases) and delivery complications: 5.55% (one case for transient uterine atony resolved with medical treatment). All the patients received folic acid and ferrous fumarate with 33.33% (6 cases) receiving antihypertensive medication (methyldopa, hydralazine, verapamil) during pregnancy (Table 1). In order of frequency indications of delivery care were: PROM: 22.23% (4 cases), PB: 22.23% (4 cases), incomplete miscarriage: 11.11% (2 cases), P-E: 11.11% (2 cases), repeated caesarean: 5.55% (1 case), SAH instability: 5.55% (1 case), AFD: 5.55% (1 case), graft function deterioration: 5.55% (1 case), low foetal reserve: 5.55% (1 case) and labour with full-term pregnancy: 5.55% (1 case). Bilateral tubular occlusion was the contraceptive method carried out in 66.67% (12) of cases and the placement of an intrauterine device in 27.78% (5 cases). In one case (5.5%) all methods were rejected. Barrier or pill methods were not prescribed.
Foetal data: Care was provided to 19 newborns (one twin pregnancy). Average weight: 2091.82g±786.65g, Apgar score (median) at one and five minutes from birth: 8-9, neonatal age: 32.83±9.02 weeks, and survival rate: 78.95% (15 cases).
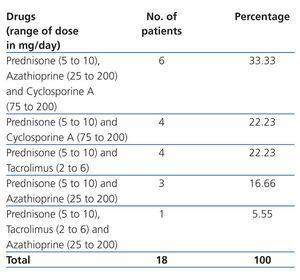
Renal Tx data: TCRF causes were: congenital renal hypoplasia: 44.45% (8 cases), primary glomerulopathy: 33.33% (6 cases), acute post-streptococcal glomerulonephritis: 11.11% (2 cases) and unknown nephropathy: 11.11% (2 cases). The majority of kidney transplants came from a LRD (44.45% [8 cases]), followed by LNRD (50% [9 cases]) and CD (5.55% [1 case]). Tx to conception time was 7.52±6.20 years (range 3 to 21). The majority were women in the first pregnancy (61.11% [11 cases]), with time from Tx to conception was 7.59±6.57 years (range 3.94 to 21); the 7 remaining patients were women who had had multiple births (range 2 to 3), with a Tx to conception time of 7.40±6.09 years (range 3 to 20.95), similar to those women on their first pregnancy (P=.95). The type and dose of immunosuppressive drug for the renal Tx is shown in Table 2. As can be observed, at the time of conception 5 women received a combination of drugs, including tacrolimus, which was changed to cyclosporine on discovery of pregnancy. The dose of other medication was not modified and in no case was the blood concentration determined. SAH frequency was 33.33% (6 cases), and 27.77% (5 cases) for abnormal proteinuria. Average prenatal enuresis was 1994.59±966.31ml/24h. Cr at first prenatal visit was 1.10±0.40, and 1.23±0.41mg/dl (P=.36) prior to delivery care. ECrC at first prenatal visit was 77.85±29.60, and 70.64±20.85ml/min/1.73m2 SC (P=.40) prior to delivery care.
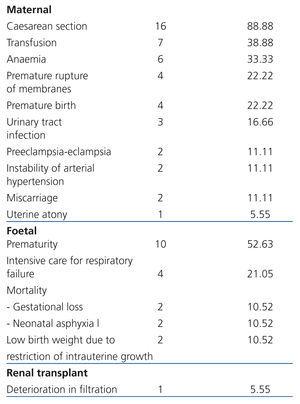
Maternal complications: In frequency order, the complications found were: caesarean section: 88.88% (16 cases), transfusion: 38.88% (7 cases), anaemia: 33.33% (6 cases), PROM: 22.22% (4 cases), PB: 22.22% (4 cases), urinary tract infection: 16.66% (3 cases), P-E: 11.11% (2 cases), SAH instability: 11.11% (2 cases), miscarriage: 11.11% (2 cases) and uterine atony: 5.55% (1 case). There were neither cases of gestational diabetes nor maternal death (Table 3).
Foetal complications: The most common complication was prematurity (52.63% [10 cases]), followed by the need for hospitalisation in the neonatal intensive care unit due to respiratory failure (21.05% [4 cases]) and low birth weight due to FGR (10.52% [2 cases]) (Table 3). Mortality was 21.05% (4 cases; 2 cases due to miscarriage [10.52%] and 2 cases by neonatal asphyxiation [10.52%]).
Renal Tx complications: ECrC was only encountered in 5.5% (1) of cases. No cases of increased proteinuria were registered, nor episodes of rejection, renal biopsy, loss of graft or need for dialysis (Table 3).
DISCUSSION
Maternal complications were much more common than foetal and renal Tx complications. The causes of maternal complications mainly affected the wellbeing of the foetus and not graft filtration, which was practically unchanged. Even when our patients were middle aged, as with the other series,20,21,25 and despite meeting the standards of current recommendations for prenatal monitoring,9-12,19-26 they were generally characterised by short pregnancies (33.11±8.72 weeks), the use of caesarean section (88.88%), premature babies (52.63%) with low weight (2091.82±786.65g) and a high foetal mortality (21.05%). Delay in patient referral to specialist care (14.35±6.74 weeks) and the elevated comorbidity at the first prenatal visit, resulting from of SAH (38.88%) and abnormal proteinuria (27.77%), possibly contributed to the presentation of maternal complications, as reported in other studies.19-21,23-25 The most common maternal complication was caesarean section: 88.88%, a significantly higher figure than that reported by Cruz et al (71.23%),21 NTPR (46 to 55%),19 UK (72%),20 Oliveira et al (61.5%)23 and Kurata et al (73.9%).24 In contrast to other cohorts, we found no urological complications of the graft due to uterine growth,28 caesarean section,29 or uterus scraping (2 cases). Other less frequent complications were: transfusion (38.88%) and anaemia (33.33%). Even though 100% of the patients received iron supplements, the percentage of anaemia was elevated. The preferred treatment for anaemia was transfusion and not administration of erythropoietin, in spite of the fact that reasonable use of this drug during gestation is considered a useful and safe resource.4
There were high percentages of complications inherent to pregnancy, including PROM (22.22%), PB (22.22%) and urinary tract infection (16.66%) and it cannot be ruled out that they have had an adverse effect on foetal condition. The same occurs with P-E and SAH instability (11.11% in both cases), whose adverse effects upon gestation have been largely documented.19-21,23-25 However, their frequency concurred with that of previous studies. Uterine atony (5.5%), the only birth complication found, was considered circumstantial. With regard to foetal complications, the increased mortality rate (21.05%), the need for the newborn to be admitted to the NICU (21.05%) and the low weight at birth due to FGR (10.52%) can be explained as secondary to the high percentage of prematurity (52.63%). Despite the majority of newborn being premature and presenting low birth weight, the survival rate (78.94%) concurred with the results of previous reports.19-21,23-25
As regards renal Tx complications, we found a reduction in ECrC in only 5.55% (1 case). No increase in proteinuria was recorded, including P-E cases, nor were there episodes of rejection, need for renal biopsy, loss of graft, or need for dialysis (Table 3). The average diuresis remained normal (1994.59±966.31ml/24h), a value that we were unable to compare, as this was not mentioned in the literature.19-21,23-25 In our patients, pregnancy did not show an adverse effect on the Tx function, which coincides with reports from at least the last decade.30-34 The concurrence of various factors could have influenced renal graft filtration, including type of donation, single Tx, the absence of previous episodes of rejection or developing rejection at the time of conception, a long time from Tx to conception, stable doses of immunosuppressive drugs, replacement of potentially aggressive medication with safer alternatives, low levels of Cr at first visit (1.10±0.40mg/dl), normal or elevated baseline ECrC (77.85±29.60 ml/min/1.73m2 SC) and satisfactory prenatal medical surveillance.30-34
In this cohort, 94.44% of the patients received a renal graft from a LRD or LNRD and only 5.56% from a DC, compared to the UK registry20 in which 80% (141 of 176 recipients) received a kidney from a DC and, in the Fernandez et al series,25 in which 65.19% (25 of 39 cases) had a DC graft. All our patients had a single renal Tx, unlike the Fernandez et al series,25 including 3 cases with two grafts and one pregnant patient transplanted on a third occasion. In our study, the time from Tx to conception was longer (7.52±6.20 years) than in the Great Britain one (6 years)20 and than that reported by Fernández et al (3.11±1.05 years).25 We had no cases of pregnancy and renal Tx ≤1 year, in contrast to that reported in the literature.9,25 Evolution of renal Tx was satisfactory both for the patients in their first delivery and those who had previously had children (P=.95), similar to the report of Ehrich et al,35 who in 1996 found that 99 out of 102 recipients of renal grafts with successful pregnancies had had two to three previous pregnancies.
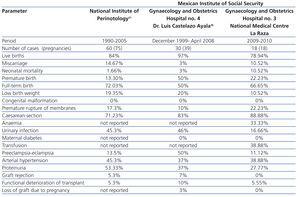
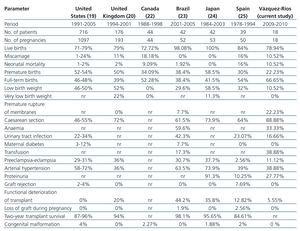
We compared our perinatal results with those of two prior national reports: the Cruz et al21 study of 60 patients that were attended to between 1990 and 2005 in the National Institute of Perinatology (INPER) and the Romero et al36 study of 30 pregnant women with renal Tx that were seen between December 1999 and April 2008 in the Gynaecology and Obstetrics Hospital No. 4 (Dr. Luis Castelazo Ayala), attached to the Mexican Institute of Social Security, both of which are tertiary care centres in Mexico City and similar to ours (Table 4). We found that our patients had similar figures in terms of miscarriages, PB, labour with full-term birth, SAH, congenital malformations and functional deterioration of the Tx. Our rates were higher in terms of caesarean sections, FGR, and neonatal mortality, and were lower in terms of living newborns, low birth weight, urinary tract infections, P-E, proteinuria, episodes of rejection and loss of Tx function. We were not able to compare our findings in terms of anaemia, transfusion and gestational diabetes as the studies did not report these figures.
In comparison with the reports from the international literature,19,20,22-25 we found that our results were similar regarding foetal survival rates, miscarriages, low and very low birth weight, neonatal mortality, SAH, P-E, proteinuria, rejection of Tx, loss of graft during pregnancy and congenital malformations. Our results were higher in terms of full-term birth, FGR, caesarean section and transfusion and lower in terms of PB, anaemia, urinary tract infection, gestational diabetes and deterioration in Tx function (Table 5).
More recently, Diaz et al37 reported on the evolution regarding gestation, graft, and the neonates of 10 pregnant patients with kidney transplants, with an average age of 28.9 years and post-transplant time of 44 months. All received immunosuppressive treatment based on prednisone 5mg/day and tacrolimus at variable doses to achieve plasmatic levels of 6 to 8ng/ml. The maternal complications included: caesarean section: 30% (3 cases), miscarriage in first trimester: 10% (1 case), SAH instability: 10% (1 case), which required the administration of a second antihypertensive drug and preeclampsia (10% [1 case]) requiring urgent caesarean section. The average weight of the newborns was 2809g and only two of them were premature, none developed important complications or malformations. The renal graft function was unaffected, proteinuria was higher in the third trimester, although it was not cause for alarm, and no episode of acute rejection was found.
The satisfactory results of this study have been thoroughly analysed by Pallardó et al38 in a review that included the analysis and comparison with available data from other studies. They concluded that the difference in frequency and distribution of the maternal, foetal and renal Tx complications between the cohorts studied can be explained based on the diversity of the characteristics of the patients and the Tx, the experience of the multidisciplinary teams, and the advances in modern immunosuppressive therapy and obstetric care in the last decades.
In this context, we agree that all the above factors as well as the fact that the first cohort began registering their patients 10 years ago or more make us consider the advisability of periodical revision of the results from specialised care centres for pregnant women with renal Tx.
CONCLUSION
In our cohort, the overall frequency of perinatal complications was elevated. Pregnancy had no adverse effect on renal Tx function. A stable renal graft in women of childbearing age is not necessarily a contraindication for pregnancy.
Conflicts of interest
The authors affirm that they have no conflicts of interest related to the content of this article.
Table 1. Antihypertensive medication administered during pregnancy
Table 2. Immunosuppressive treatment during pregnancy
Table 3. Perinatal complications
Table 4. Comparison of perinatal complications with previous reports from two tertiary care centres in Mexico City
Table 5. Comparison of frequency of perinatal complications with that reported in global literature