To the Editor,
Multiple myeloma (MM) is a treatable, although incurable disease.1-3 Its prognosis has improved during recent years, with an average survival of 2-3 years, given a therapeutic change with the advent of autologous haematopoietic progenitor cell transplantation (AHPCT) and three new myeloma drugs: thalidomide, lenalidomide and bortezomib,4 which have proven to be safe for patients with MM and renal failure.1
Renal failure is one of the main factors associated with the disease’s poor prognosis.2 I develops in 25%-30% of cases and 2%-3% of them need dialysis, with an average survival rate of between 4 months to a year.3,4 It has a multifactorial origin, although its most frequent cause is the elimination of light chains (Bence-Jones proteinuria). When it is present in tubules is histologically called “myeloma kidney”.1
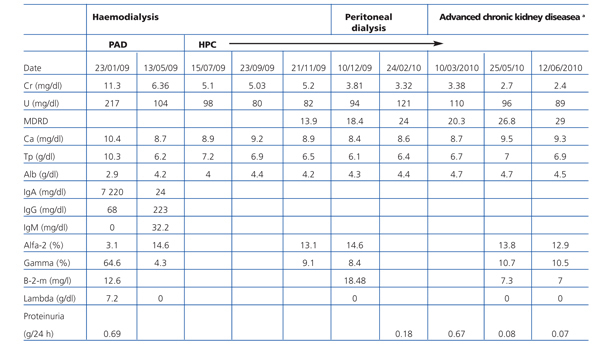
We report the case of a 53-year-old patient, diagnosed with MM IgA lambda stage IIIB according to the Durie Salmon staging system. He presented with acute renal failure, likely to be secondary to myeloma kidney. He was indicated haemodialysis at diagnosis. He started front line chemotherapy with bortezomib, adriamycin and dexamethasone (PAD combination therapy) prior to AHPCT with melphalan, with excellent clinical tolerance, which allowed him to achieve complete remission (CR). However, he had no kidney response. Eleven months after starting the dialysis programme, the patient presented with an improved glomerular filtration rate (GFR) and was able to stop dialysis treatment definitively 14 months after diagnosis (Table 1).
The degree of renal dysfunction has an impact on MM patients’ prognosis, especially in those that need dialysis, reporting lower survival rates, higher short-term mortality (estimated 4 months), greater susceptibility to infections, longer hospital stays and greater compromise to the patient’s quality of life. High-dose chemotherapy and AHPCT have traditionally been contraindicated for all of these reasons.5 However, the treatment of choice is polychemotherapy in patients under 65 years with a generally good condition. As well as dexamethasone, regimens include vincristine, adriamycin and cyclophosphamide, together with new myelomatosis drugs: thalidomide, lenalidomide and bortezomib, followed by AHPCT,5,6 as well as dialysis support when necessary, given that it increases the likelihood of CR for 20%-40% of cases. It has a progression-free survival of 2.5-4 years and a general survival of 4-5 years,7-9 with total or partial GFR recovery in 25%-58%, which entails improved survival.4,10
Tauro et al, and other authors, have reported that patients who receive chemotherapy and AHPCTs can obtain partial kidney function recovery, reducing their dialysis dose and frequency. However, very few patients are able to definitively stop dialysis following treatment. They also describe that the type of paraprotein used (IgA and IgM are associated with a higher risk of progression than IgG), MM evolution time, and renal failure evolution time influence partial kidney function recovery.4,10,11
Badros et al conducted the first prospective study on MM patients undergoing AHPCT. They reported that patients under 70 years old with MM and renal failure including those on dialysis, should be treated with lower chemotherapy doses (melphalan at 140mg instead of 200mg), as it reduces the incidence of adverse effects. They also describe early AHPCT as being a safe treatment, which increases the likelihood of CR, total or partial GFR recovery, and therefore, overall survival. However, results show that 12 months after renal failure, GFR recovery is highly unlikely.4
Matsue recently published a study on the possibility of reversing dialysis-dependent renal failure, and its relation to light chains. This study considered high-dose dexamethasone to be the gold standard treatment for patients with MM and renal failure, given its rapid response, and that it should be co-administered with new drugs such as thalidomide and bortezomib, which have proven to be greatly effective at treating MM associated with GFR deterioration. In their study, 67% of patients who had been undergoing dialysis for 2 months stopped dialysis following treatment with dexamethasone and thalidomide. Bortezomib was used successfully as a second-line treatment for 3 dialysis-dependent patients, and did not show any major adverse effects. They also observed a greater survival in those patients who had been undergoing dialysis for less than 4 months, and long-term survival similar to patients without renal failure. GFR recovery was therefore associated with a reduction in light chain serum concentration for all patients included in the study. An excess of light chains synthesised by myeloma cells is the main cause of renal function deterioration, as they accumulate in the renal tubules. Rapidly reducing them is therefore of utmost importance for recovering kidney function.12
To conclude, our case reported a patient under 65 years of age, diagnosed with MM IgA stage IIIB (and therefore with greater risk of progression), with severe renal failure at the time of diagnosis and who needed dialysis (low survival and high morbidity and mortality). He obtained CR but had no kidney response. He was indicated a PAD combination regimen and AHPCT + melphalan. He presented with gradual GFR progression a year after diagnosis, and stopped dialysis at 14 months (which is an exceptional case as we have seen in the medical literature review). We are therefore able to conclude that a safe and effective treatment to ensure remission in dialysis-dependent patients is the induction regimen with dexamethasone, alongside a myeloma drug such as bortezomib, before AHPCT and low doses of melphalan, which in addition to a gradual reduction in light chain formation and excretion, promotes GFR recovery, and improves long-term patient survival.
Table 1. Analytical evolution