There association between the development of vasculitis associated with neutrophil anti-cytoplasmic antibodies (ANCA) and cancer has been shown previously. In 1993 there were described, the first 4 cases of patients presenting with lung or bladder carcinoma shortly after the clinical onset of ANCA positive glomerulonephritis.1 Since then, few more clinical cases and some evidence of such association have been shown in patients registries with positive ANCA, both c-ANCA and p-ANCA2–5 measured by indirect immunofluorescence or direct ELISAs, which had a very low sensitivity and specificity as compared with the current methods.
We present the case of a 48-year-old male smoker, who started with malaise and the blood chemistry (Table 1) showed a reduction of renal function (creatinine [Cr]: 1.8mg/dl; glomerular filtration rate [GFR]: 42ml/min); urine sediment: proteinuria ++++, hemoglobin ++++ and >100 red blood cells per field (60% dysmorphic). Proteinuria: 3900mg/24h and a blood sedimentation rate of 100mm. The immunological study showed that immunoglobulins and complement were normal, antinuclear antibodies with homogeneous pattern at 1/640 with no specificity and antimyeloperoxidase antibodies (MPO) >600IU/ml; Antiproteinase 3 antibodies, glomerular basement membrane antibody and rheumatoid factor were negative. Urological ultrasound and chest X-ray without relevant findings. Renal biopsy showed 22 glomeruli, 2 of them sclerosed, 13 with extracapillary proliferation, 3 with fibrous crescent and 4 with incipient lesions of glomerular necrosis. In addition, there was a moderate dispersed interstitial infiltrate of lymphocytes, polymorphonuclear cells and plasma cells. The arteries had no significant lesions and by immunofluorescence there were focal and segmental distribution of C3 (++). Treatment with 500mg of methylprednisolone was initiated on consecutive days, followed by oral prednisone at 1mg/kg, with subsequent tapering plus intravenous cyclophosphamide at doses of 1g/m2/month.
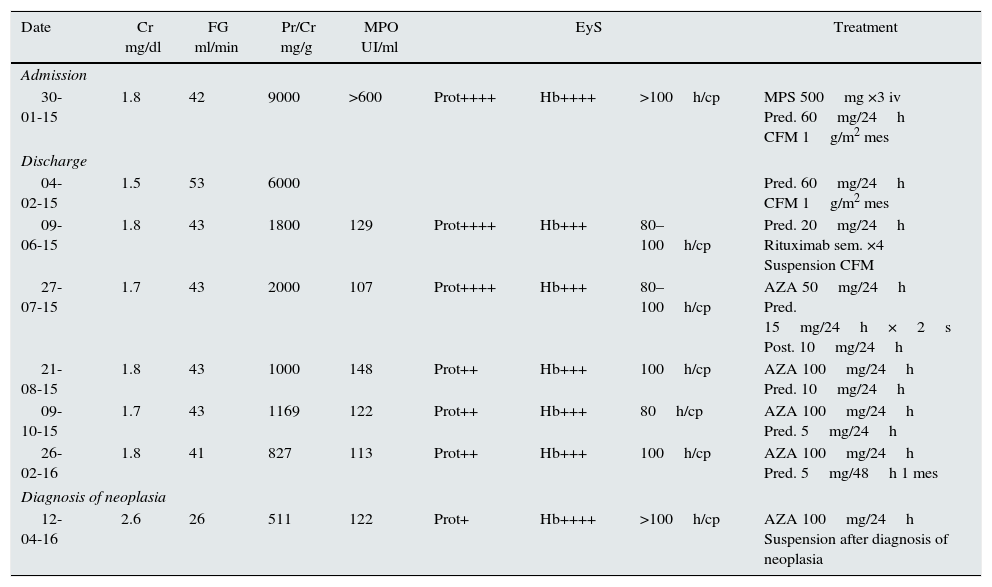
Evolution of the main findings in the blood chemistry and the administered treatment.
| Date | Cr mg/dl | FG ml/min | Pr/Cr mg/g | MPO UI/ml | EyS | Treatment | ||
|---|---|---|---|---|---|---|---|---|
| Admission | ||||||||
| 30-01-15 | 1.8 | 42 | 9000 | >600 | Prot++++ | Hb++++ | >100h/cp | MPS 500mg ×3 iv Pred. 60mg/24h CFM 1g/m2 mes |
| Discharge | ||||||||
| 04-02-15 | 1.5 | 53 | 6000 | Pred. 60mg/24h CFM 1g/m2 mes | ||||
| 09-06-15 | 1.8 | 43 | 1800 | 129 | Prot++++ | Hb+++ | 80–100h/cp | Pred. 20mg/24h Rituximab sem. ×4 Suspension CFM |
| 27-07-15 | 1.7 | 43 | 2000 | 107 | Prot++++ | Hb+++ | 80–100h/cp | AZA 50mg/24h Pred. 15mg/24h×2s Post. 10mg/24h |
| 21-08-15 | 1.8 | 43 | 1000 | 148 | Prot++ | Hb+++ | 100h/cp | AZA 100mg/24h Pred. 10mg/24h |
| 09-10-15 | 1.7 | 43 | 1169 | 122 | Prot++ | Hb+++ | 80h/cp | AZA 100mg/24h Pred. 5mg/24h |
| 26-02-16 | 1.8 | 41 | 827 | 113 | Prot++ | Hb+++ | 100h/cp | AZA 100mg/24h Pred. 5mg/48h 1 mes |
| Diagnosis of neoplasia | ||||||||
| 12-04-16 | 2.6 | 26 | 511 | 122 | Prot+ | Hb++++ | >100h/cp | AZA 100mg/24h Suspension after diagnosis of neoplasia |
AZA: azathioprine; CFM: cyclophosphamide; Cr: serum creatinine; EyS; elemental and sediment; FG: glomerular filtration by the formula CKD-EPI; Hb: hemoglobin; Pred: prednisone; Pr/Cr: protein/creatinine ratio in urine.
After five months, the renal function did not show any improvement (Cr 1.8mg/dl and GFR 43.5ml/min) and the urine sediment remained active (Table 1) with positive MPO. Then, it was decided to discontinue cyclophosphamide and start treatment with rituximab.
After 4 doses of rituximab, the Cr was 1.2mg/dl, the GFR 63ml/min, Pr/Cr 2727mg/g. Sediment: protein: ++++, hemoglobin: +++, 40–50 red blood cells/field and MPO 130IU/ml. Then, maintenance therapy with azathioprine was initiated.
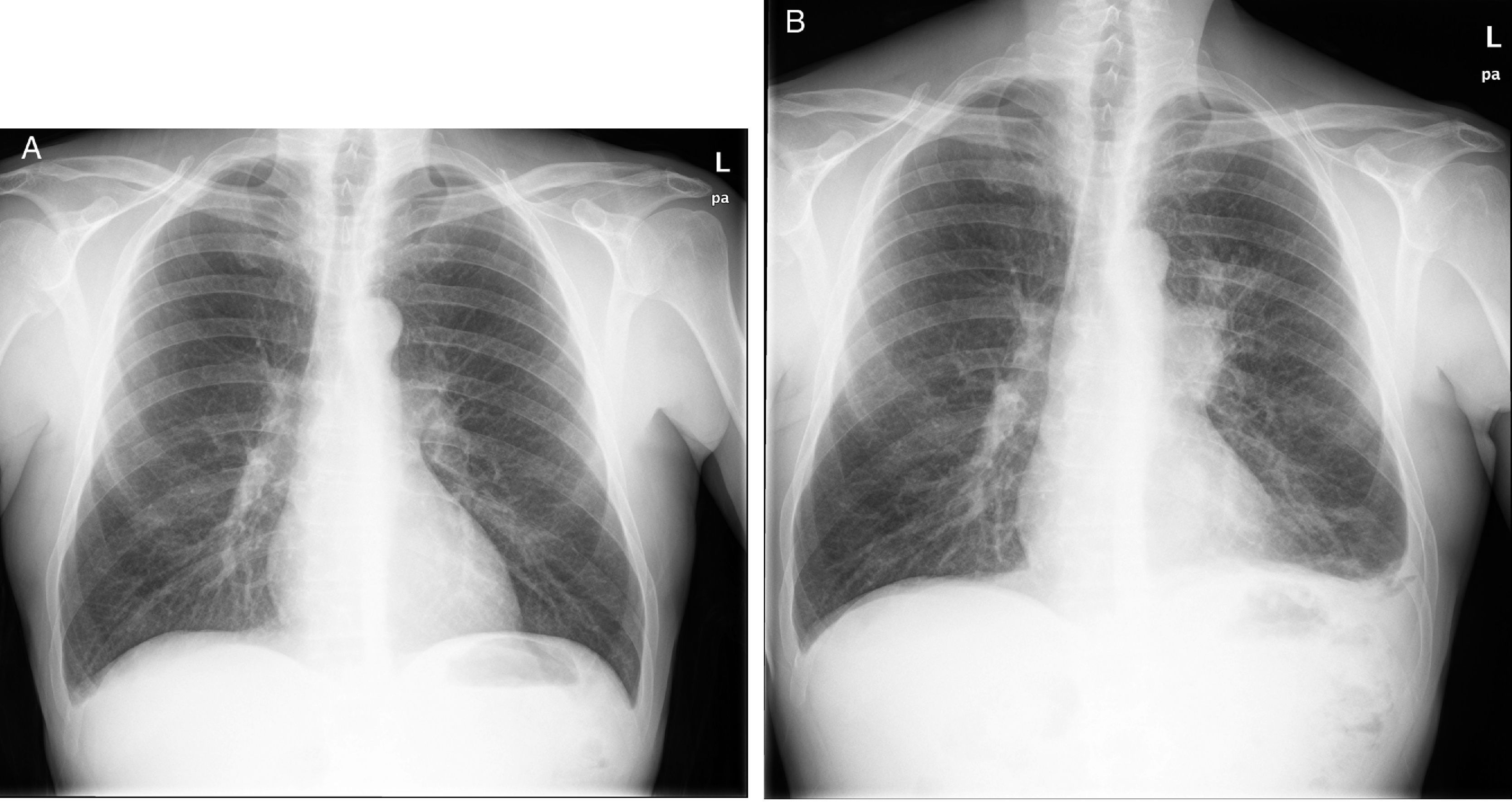
Nine months later he developed cough with expectoration that did not improve with antibiotics. A left hiliar mass was found in the chest X-ray; he underwent transbronchial biopsy that revealed small cell carcinoma. The CT showed multiple pleural, hepatic tumor implants and adenopathic paratracheal and subcarinal conglomerates (Fig. 1). Faced with this diagnosis, immunosuppression was withdrawn and treatment with carboplatin-etoposide was initiated. However, the patients suffered from a progressive clinical deterioration and death one month later.
The case presented is one more of the few cases of ANCA as a paraneoplastic marker. The patient, although with a history of smoking, did not present evidence of lung carcinoma when glomerulonephritis was diagnosed. The presence of elevated MPO titers, which remained positive at considerable high values, together with active urinary sediment, led to prescribe an increase in immunosuppression that included rituximab and, subsequently, azathioprine. Thereafter, the patient presented a lung carcinoma. During the evolution of the patient, attention was drawn to the fact that the MPO titers were >600IU/ml at the beginning of glomerulonephritis and with the initial treatment the value was reduced to fell to 120U/ml, but always remained at these high levels independently of immunosuppression treatment that was added. The method used to detect antibodies is anchoring, which increases sensitivity (50%) and maintains a specificity greater than 99%. However, maintenance of MPO titers in the described clinical case may reflect a paraneoplastic effect, since ANCAs have also been described as paraneoplastic autoantibodies both in solid organ tumors and in hematological neoplasms.6 It is possible that dysregulation of the immune system causing vasculitis is the basis of the subsequent development of a neoplasm by favoring tumor escape from the mechanisms of immunovigilance. However, it can not be ruled out that the tumor process was already underway in a middle-aged smoker patient and that cell destruction induced epitopes that trigger the humoral response of MPO.7 Treatment with rituximab probably precipitated the development of the disease. In fact, the patient had antinuclear antibodies at the onset of renal failure, which has also been associated with the exposure of neoepitope in the context of cellular destruction in inflammatory processes.8
In conclusion, the present case reflects the importance of considering the possibility of the development of neoplasias in an ANCA glomerulonephritis in which the management of immunosuppression should be exquisite, especially in the case of high titers of autoantibodies.
Please cite this article as: Belmar L, López-Hoyos M, Irure J, Rodrigo E, Martin de Francisco ÁL, Fernández-Fresnedo G. Glomerulonefritis pauciinmune paraneoplásica en paciente con carcinoma pulmonar. Nefrologia. 2017;37:539–541.