Membranous glomerulonephritis (MGN) is an autoimmune disease characterised by subepithelial immune complex deposits.1,2 Approximately 75% of MGN cases are primary or idiopathic, with the rest being secondary forms associated with systemic diseases, infections, cancer and drugs.2 Among adult patients with primary MGN, 70–80% have circulating antibodies to the M-type receptor of phospholipase A2 (anti-PLA2R), located on the podocyte membrane. Not described in other glomerular diseases, these antibodies have diagnostic value for primary MGN, and correlate with clinical activity and response to treatment.3,4
We describe a patient with MGN who had tested positive for anti-PLA2R using two techniques with a histological pattern suggesting a secondary cause, probably lupus.
This was a 77-year-old male referred for nephrotic syndrome. His medical history included a 40–50-year history of epilepsy, on treatment with diphenylhydantoin. Eight months earlier he had begun to have lower limb oedema and raised blood pressure. He reported nocturia 2–3 times a night, with no other systemic symptoms, and was on treatment with diphenylhydantoin, amlodipine, furosemide and enoxaparin. Physical examination revealed: blood pressure 143/86mmHg and oedema of the lower limbs with fovea to the knees; the rest of the examination was unremarkable.
Laboratory tests found: haemoglobin 10.3g/dL; leukocytes 6310/mm3 (lymphocytes 1300/mm3); MCV and MCHC normal; platelets 276,000; iron 82mcg/dL; ferritin 29.2ng/mL; direct Coombs negative; urea 32mg/dL; creatinine 1.20mg/dL; albumin 2.3g/dL; coagulation, electrolytes and rest of clinical biochemistry trace elements were normal. IgG was 461mg/dL, IgM 34mg/dL, and IgA normal. High resolution serum protein electrophoresis: weak monoclonal IgG kappa component (kappa chains 52.6mg/L, lambda chains 27.2mg/L, kappa/lambda ratio 1.94). Anti-PLA2R antibodies were positive by indirect immunofluorescence and by ELISA 274U/mL (reference value <20), (indirect immunofluorescence and ELISA: Medizinische Labordiagnostika, EUROINMUN AG, Luebeck, Germany). Immunology tests: ANA+ 1/1280; C3, C4, anti-DNA, anti-histones, ENA, anti-cardiolipin antibodies, ANCA, anti-TPO and cryoglobulins normal/negative. Angiotensin converting enzyme, thyroid hormones and PSA were normal. Urine: proteinuria 8.920g/24h (high resolution electrophoresis: non-selective glomerular pattern, weak monoclonal IgG kappa component). Urine culture was negative. Mantoux and QuantiFERON were also negative. Serology was negative for HBV, HCV, HIV, Parvovirus B19 and CMV; EBV infection in the past. HBV PCR and HCV PCR were negative. RPR was negative.
No abnormalities were observed on chest X-ray or echocardiogram; chest–abdomen–pelvis CT found bilateral lamellar pleural effusion, with no other abnormalities; MRI of renal veins found no signs of thrombosis; gastro/colonoscopy: colon polyps detected and resected (biopsy: tubular adenomas with low grade dysplasia).
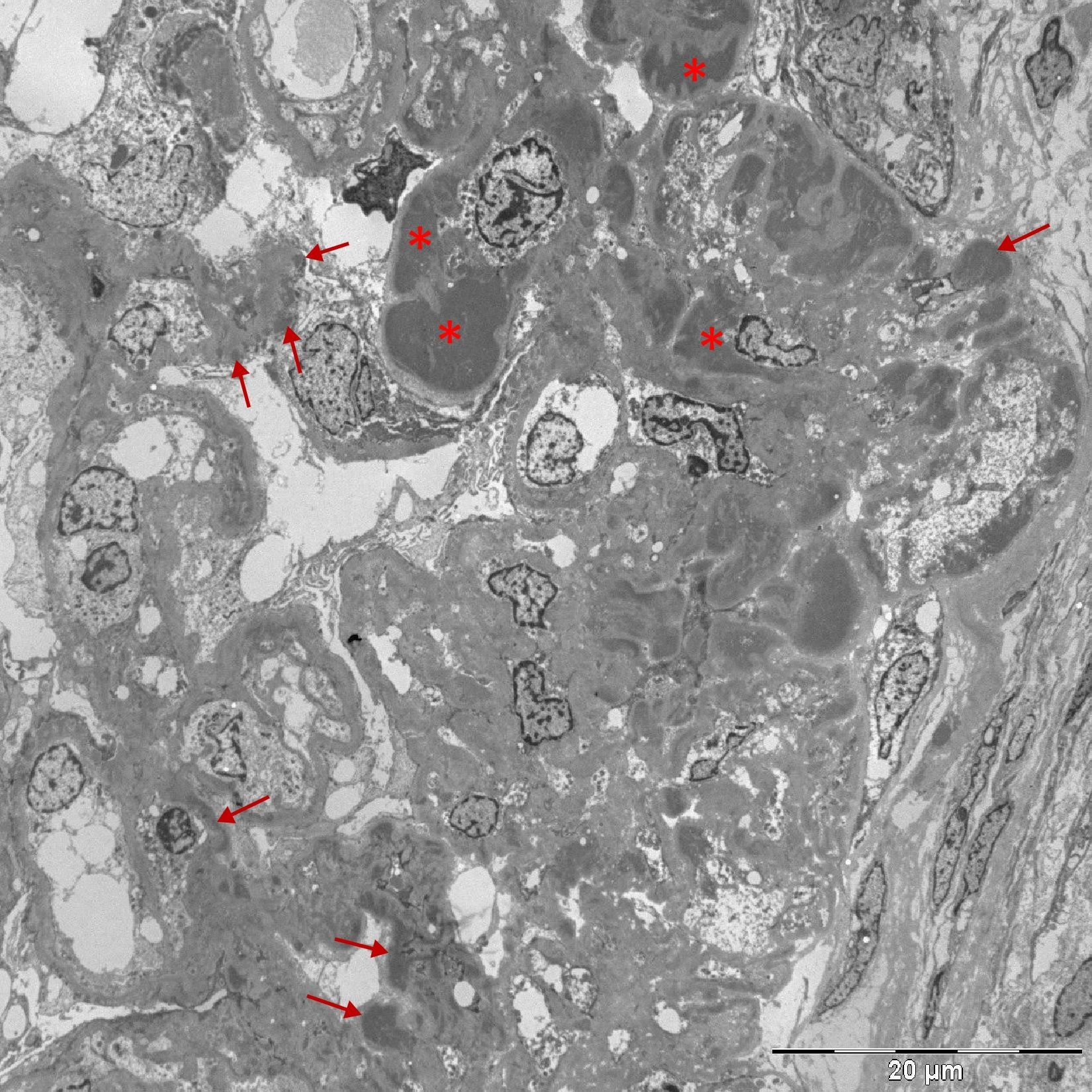
Percutaneous renal biopsy was performed (18 glomeruli, 2 of them sclerotic), showing thickening of basement membranes with vacuolated appearance and mesangial proliferation; immunofluorescence revealed IgG and C3 with diffuse granular pattern in the capillary walls and mesangium, C1q negative, kappa and lambda positive (+++); the electron microscopy study showed massive subendothelial, subepithelial and mesangial deposits and podocyte fusion (Fig. 1). Taken as a whole, the picture was consistent with secondary MGN.
The patient was prepared for sedated colonoscopy with senna and saline enema. During the study, he developed hypotension and his creatinine rose to 1.69mg/dL. ACE inhibitor/ARA2 treatment were not used, at least initially, and the patient was treated with prednisone and mycophenolate mofetil.
Our first diagnostic impression was primary MGN. However, the presence of mesangial proliferation and subendothelial and mesangial deposits suggested a secondary form of MGN. Obviously hepatitis B and C and cancer were ruled out. There was no light chain restriction, so it did not appear to be a monoclonal gammapathy with renal involvement. We believe that our patient may have latent systemic lupus erythematosus (SLE). Lupus MGN can present with few clinical and serological manifestations of this disease.5 Nevertheless, this patient could be diagnosed with SLE according to SLICC criteria.6 It seems unlikely that diphenylhydantoin played any role, because nephropathy is rare in drug-induced lupus and anti-histone antibodies are usually positive.
Anti-PLA2R antibodies are a useful biomarker for the diagnosis of primary MGN; studies have shown anti-PLA2R detection techniques to have a sensitivity of 94.4% (indirect immunofluorescence) and 97.2% (ELISA), and a specificity of 100%.7 Positive anti-PLA2R has been described in membranous nephropathy secondary to cancer, lupus, hepatitis B and sarcoidosis.1 Qin et al.8 studied 20 patients with lupus membranous nephropathy, one of whom was positive for anti-PLA2R. Another study9 included 25 patients of predominantly European origin diagnosed with lupus membranous nephropathy; anti-PLA2R was not detected in any of them. Anti-PLA2R positivity in lupus MGN is therefore uncommon, of doubtful significance and may merely be a coincidence. However, in membranous nephropathy secondary to HBV and sarcoidosis with positive anti-PLA2R, these antibodies may play a pathogenic role.10,11
It has been suggested that a diagnosis of MGN can be assumed in nephrotic syndrome with positive anti-PLA2R, especially in cases of risk for renal biopsy.1 As this patient illustrates, unless there is a serious contraindication, the biopsy provides additional information.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To Dr. Jerónimo Forteza (Mixed Molecular Pathology Unit, Valencian Institute of Pathology, Universidad Católica de Valencia) for the electron microscopy study.
Please cite this article as: Alvarado R, Enríquez R, Muci T, Sirvent AE, Lozano Vera V, Millán I, et al. Síndrome nefrótico, anticuerpos anti-PLA2R y glomerulonefritis membranosa. ¿Es necesaria la biopsia renal?. Nefrología. 2017;37:447–449.