Content
The nail-patella syndrome is an uncommon hereditary disease caused by mutations that result in a loss of function of the transcription factor LMX1B. Prevalence is estimated at 1/50,000 new-born babies. It is transmitted with an autosomal dominant inheritance pattern with complete penetrance. The pleiotropic gene LMX1B, a member of the “homeogene” family involved in development, participates in the normal configuration of the dorsal-ventral axis and the glomerular basement membrane during embryonic development.
The disease was first described by Little in 1897,1 although Fong wrote the classic description of the disease in 1946.2 The link with kidney disease was established by Hawkins and Smith in 1950.3 The gene responsible was described by Dreyer et al. in 1998.4
The extent of severity of the clinical phenotype is extremely variable. Basically, it is an illness that affects the nails, skeletal system, kidneys and eyes. The clinical manifestations that define the disease are grouped in the clinical tetrad consisting of nail dysplasia (98%), hypoplasia and aplasia of the patella (74 %), functional limitation of elbows (70%) and the presence of iliac horns (70 %); the latter, which are pathognomonic, can be observed by ultrasound scan from the third trimester of pregnancy. Around 40% of patients show renal involvement consisting of haematuria and proteinuria. 5-10% develop nephrotic range proteinuria in childhood or adolescence and progress to terminal renal failure within varying periods of time. With the electron microscope, we can see the podocyte foot process effacement, glomerular basement membrane thickening with areas of rarefaction and dispersed collagen fibril deposits, which are more numerous in the mesangial matrix. The transcription factor LMX1B is expressed in the podocyte in postnatal life, which suggests that its role is to regulate various genes in this cell, such as NPHS2 and CD2AP.5
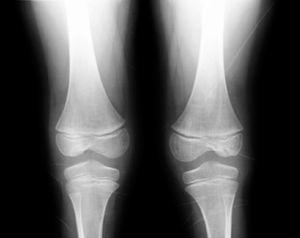
We had the opportunity to study a girl of 8 years and 3 months of age who was referred due to proteinuria detection. At birth, a low weight was observed, along with the presence of dysmorphic features, such as bowlegs, mild arthrogryposis in her elbows, macrocephaly and nail hypoplasia. She started to walk at 21 months due to hip dysplasia. She underwent several surgeries to correct skeletal defects. In the current clinical examination she displayed short stature, macrocephaly with a very prominent forehead, synophrys and very thick eyebrows, a broad nasal philtrum with a thin upper lip, down-slanting palpebral fissures, anteverted nostrils, hypertelorism, absence of both patellas (Figure 1) and finger nail hypoplasia with dystrophic nails (Figure 2). Oedemas were not observed. She is the second daughter of non-consanguineous parents with no history of renal disease in any of the family branches. Her father has mild abnormalities in his nails. Biochemistry work-up found nephrotic range proteinuria (7.3g/l protein/creatinine [Cr] ratio: 6.73mg/mg), hypoproteinaemia (5.3g/l), reduced levels of IgG (512mg/dl) and hypercholesterolaemia (312mg/dl). The glomerular filtration rate (GFR) was normal (134ml/min/1.73m2). The LMX1B gene study showed mutation c.728G>C (p.Trp243Ser) in heterozygosis. This mutation has previously been described in literature. Since LMX1B gene mutations have only been associated with the nail-patella syndrome, we assume that it is responsible for the patient’s clinical profile. In the genetic study carried out on first degree relatives, the mutation was not observed, and as such, this must be a de novo mutation. The patient was treated with oral enalapril, in an attempt to reduce proteinuria, and with simvastatin. With this treatment, proteinuria decreased to 5.35g/l (protein/Cr ratio: 2.84mg/mg), GFR remained normal and the patient remained free of oedemas.
In this disease, more than 130 different mutations have been described, mainly consisting of changes in just one nucleotide. They are predominantly distributed between exons 2 and 6. A more common series of mutations has been described which together represent 30% of the total. 12% of mutations are de novo.7 No phenotype-genotype correlation has been established, and as such, although prenatal diagnosis can be carried out, there is marked inter and intrafamilial variability.7 The confirmation of a mutation in the LMX1B gene avoids the need to perform a renal biopsy to confirm diagnosis. There is no specific treatment. There is no recurrence of lesions of the glomerular basement membrane after renal transplantation.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Absence of patellas
Figure 2. Nail hypoplasia and dystrophic nails